A retinoic acid-dependent stroma-leukemia crosstalk promotes chronic lymphocytic leukemia progression
- PMID: 29725010
- PMCID: PMC5934403
- DOI: 10.1038/s41467-018-04150-7
A retinoic acid-dependent stroma-leukemia crosstalk promotes chronic lymphocytic leukemia progression
Abstract
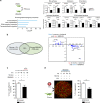
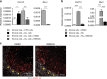
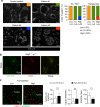
In chronic lymphocytic leukemia (CLL), the non-hematopoietic stromal microenvironment plays a critical role in promoting tumor cell recruitment, activation, survival, and expansion. However, the nature of the stromal cells and molecular pathways involved remain largely unknown. Here, we demonstrate that leukemic B lymphocytes induce the activation of retinoid acid synthesis and signaling in the microenvironment. Inhibition of RA-signaling in stromal cells causes deregulation of genes associated with adhesion, tissue organization and chemokine secretion including the B-cell chemokine CXCL13. Notably, reducing retinoic acid precursors from the diet or inhibiting RA-signaling through retinoid-antagonist therapy prolong survival by preventing dissemination of leukemia cells into lymphoid tissues. Furthermore, mouse and human leukemia cells could be distinguished from normal B-cells by their increased expression of Rarγ2 and RXRα, respectively. These findings establish a role for retinoids in murine CLL pathogenesis, and provide new therapeutic strategies to target the microenvironment and to control disease progression.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Inhibition of BCR signaling using the Syk inhibitor TAK-659 prevents stroma-mediated signaling in chronic lymphocytic leukemia cells.Oncotarget. 2017 Jan 3;8(1):742-756. doi: 10.18632/oncotarget.13557. Oncotarget. 2017. PMID: 27888629 Free PMC article.
-
Access to follicular dendritic cells is a pivotal step in murine chronic lymphocytic leukemia B-cell activation and proliferation.Cancer Discov. 2014 Dec;4(12):1448-65. doi: 10.1158/2159-8290.CD-14-0096. Epub 2014 Sep 24. Cancer Discov. 2014. PMID: 25252690
-
HIF-1α regulates the interaction of chronic lymphocytic leukemia cells with the tumor microenvironment.Blood. 2016 Apr 21;127(16):1987-97. doi: 10.1182/blood-2015-07-657056. Epub 2016 Jan 29. Blood. 2016. PMID: 26825709 Free PMC article.
-
Survival and Immunosuppression Induced by Hepatocyte Growth Factor in Chronic Lymphocytic Leukemia.Curr Mol Med. 2017;17(1):24-33. doi: 10.2174/1566524017666170220095838. Curr Mol Med. 2017. PMID: 28231754 Review.
-
The chronic lymphocytic leukemia microenvironment: Beyond the B-cell receptor.Best Pract Res Clin Haematol. 2016 Mar;29(1):40-53. doi: 10.1016/j.beha.2016.08.007. Epub 2016 Aug 11. Best Pract Res Clin Haematol. 2016. PMID: 27742071 Review.
Cited by
-
Retinoic Acid, Leaky Gut, and Autoimmune Diseases.Nutrients. 2018 Aug 3;10(8):1016. doi: 10.3390/nu10081016. Nutrients. 2018. PMID: 30081517 Free PMC article. Review.
-
Mouse models of chronic lymphocytic leukemia and Richter transformation: what we have learnt and what we are missing.Front Immunol. 2024 Jun 6;15:1376660. doi: 10.3389/fimmu.2024.1376660. eCollection 2024. Front Immunol. 2024. PMID: 38903501 Free PMC article. Review.
-
Understanding CLL biology through mouse models of human genetics.Blood. 2021 Dec 23;138(25):2621-2631. doi: 10.1182/blood.2021011993. Blood. 2021. PMID: 34940815 Free PMC article. Review.
-
Retinoic Acid Receptors in Acute Myeloid Leukemia Therapy.Cancers (Basel). 2019 Dec 1;11(12):1915. doi: 10.3390/cancers11121915. Cancers (Basel). 2019. PMID: 31805753 Free PMC article. Review.
-
The Calcitriol/Vitamin D Receptor System Regulates Key Immune Signaling Pathways in Chronic Lymphocytic Leukemia.Cancers (Basel). 2021 Jan 14;13(2):285. doi: 10.3390/cancers13020285. Cancers (Basel). 2021. PMID: 33466695 Free PMC article.
References
-
- Bagdi E, Krenacs L, Krenacs T, Miller K, Isaacson PG. Follicular dendritic cells in reactive and neoplastic lymphoid tissues: a reevaluation of staining patterns of CD21, CD23, and CD35 antibodies in paraffin sections after wet heat-induced epitope retrieval. Appl. Immunohistochem. Mol. Morphol. 2001;9:117–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

