Patient derived xenografts (PDX) predict an effective heparanase-based therapy for lung cancer
- PMID: 29721203
- PMCID: PMC5922397
- DOI: 10.18632/oncotarget.25022
Patient derived xenografts (PDX) predict an effective heparanase-based therapy for lung cancer
Abstract
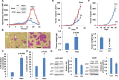
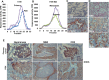
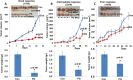
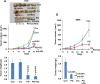
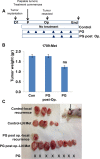
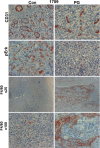
Heparanase, the sole heparan sulfate (HS) degrading endoglycosidase, regulates multiple biological activities that enhance tumor growth, metastasis, angiogenesis, and inflammation. Heparanase accomplishes this by degrading HS and thereby facilitating cell invasion and regulating the bioavailability of heparin-binding proteins. HS mimicking compounds that inhibit heparanase enzymatic activity were examined in numerous preclinical cancer models. While these studies utilized established tumor cell lines, the current study utilized, for the first time, patient-derived xenografts (PDX) which better resemble the behavior and drug responsiveness of a given cancer patient. We have previously shown that heparanase levels are substantially elevated in lung cancer, correlating with reduced patients survival. Applying patient-derived lung cancer xenografts and a potent inhibitor of heparanase enzymatic activity (PG545) we investigated the significance of heparanase in the pathogenesis of lung cancer. PG545 was highly effective in lung cancer PDX, inhibiting tumor growth in >85% of the cases. Importantly, we show that PG545 was highly effective in PDX that did not respond to conventional chemotherapy (cisplatin) and vice versa. Moreover, we show that spontaneous metastasis to lymph nodes is markedly inhibited by PG545 but not by cisplatin. These results reflect the variability among patients and strongly imply that PG545 can be applied for lung cancer therapy in a personalized manner where conventional chemotherapy fails, thus highlighting the potential benefits of developing anti-heparanase treatment modalities for oncology.
Keywords: PDX; PG545; heparanase; lung cancer; metastasis.
Conflict of interest statement
CONFLICTS OF INTEREST Edward Hammond is employed by Zucero Therapeutics, Darra, Queensland, Australia. All other authors have no potential conflicts of interest to declare.
Figures
Similar articles
-
Heparanase inhibitors restrain mesothelioma.Oncotarget. 2018 Dec 7;9(96):36830-36832. doi: 10.18632/oncotarget.26243. eCollection 2018 Dec 7. Oncotarget. 2018. PMID: 30627323 Free PMC article.
-
PG545, a heparan sulfate mimetic, reduces heparanase expression in vivo, blocks spontaneous metastases and enhances overall survival in the 4T1 breast carcinoma model.PLoS One. 2012;7(12):e52175. doi: 10.1371/journal.pone.0052175. Epub 2012 Dec 26. PLoS One. 2012. PMID: 23300607 Free PMC article.
-
Heparanase: From basic research to therapeutic applications in cancer and inflammation.Drug Resist Updat. 2016 Nov;29:54-75. doi: 10.1016/j.drup.2016.10.001. Epub 2016 Oct 6. Drug Resist Updat. 2016. PMID: 27912844 Free PMC article. Review.
-
The heparanase inhibitor PG545 is a potent anti-lymphoma drug: Mode of action.Matrix Biol. 2019 Apr;77:58-72. doi: 10.1016/j.matbio.2018.08.005. Epub 2018 Aug 7. Matrix Biol. 2019. PMID: 30096360
-
Opposing Functions of Heparanase-1 and Heparanase-2 in Cancer Progression.Trends Biochem Sci. 2018 Jan;43(1):18-31. doi: 10.1016/j.tibs.2017.10.007. Epub 2017 Nov 20. Trends Biochem Sci. 2018. PMID: 29162390 Free PMC article. Review.
Cited by
-
A New Synthesized Dicarboxylated Oxy-Heparin Efficiently Attenuates Tumor Growth and Metastasis.Cells. 2024 Jan 23;13(3):211. doi: 10.3390/cells13030211. Cells. 2024. PMID: 38334603 Free PMC article.
-
Cancer Metastasis: The Role of the Extracellular Matrix and the Heparan Sulfate Proteoglycan Perlecan.Front Oncol. 2020 Jan 17;9:1482. doi: 10.3389/fonc.2019.01482. eCollection 2019. Front Oncol. 2020. PMID: 32010611 Free PMC article. Review.
-
Application of Animal Models in Cancer Research: Recent Progress and Future Prospects.Cancer Manag Res. 2021 Mar 15;13:2455-2475. doi: 10.2147/CMAR.S302565. eCollection 2021. Cancer Manag Res. 2021. PMID: 33758544 Free PMC article. Review.
-
Elucidating the Consequences of Heparan Sulfate Binding by Heparanase 2.Front Oncol. 2021 Jan 29;10:627463. doi: 10.3389/fonc.2020.627463. eCollection 2020. Front Oncol. 2021. PMID: 33585253 Free PMC article.
-
Targeting Heparanase in Cancer: Inhibition by Synthetic, Chemically Modified, and Natural Compounds.iScience. 2019 May 31;15:360-390. doi: 10.1016/j.isci.2019.04.034. Epub 2019 May 3. iScience. 2019. PMID: 31103854 Free PMC article. Review.
References
-
- Vlodavsky I, Gross-Cohen M, Weissmann M, Ilan N, Sanderson RD. Opposing Functions of Heparanase-1 and Heparanase-2 in Cancer Progression. Trends Biochem Sci. 2018;43:18–31. https://doi.org/10.1016/j.tibs.2017.10.007. - DOI - PMC - PubMed
-
- Vlodavsky I, Folkman J, Sullivan R, Fridman R, Ishai-Michaeli R, Sasse J, Klagsbrun M. Endothelial cell-derived basic fibroblast growth factor: synthesis and deposition into subendothelial extracellular matrix. Proc Natl Acad Sci U S A. 1987;84:2292–96. https://doi.org/10.1073/pnas.84.8.2292. - DOI - PMC - PubMed
-
- Dempsey LA, Brunn GJ, Platt JL. Heparanase, a potential regulator of cell-matrix interactions. Trends Biochem Sci. 2000;25:349–51. https://doi.org/10.1016/S0968-0004(00)01619-4. - DOI - PubMed
-
- Vlodavsky I, Singh P, Boyango I, Gutter-Kapon L, Elkin M, Sanderson RD, Ilan N. Heparanase: from basic research to therapeutic applications in cancer and inflammation. Drug Resist Updat. 2016;29:54–75. https://doi.org/10.1016/j.drup.2016.10.001. - DOI - PMC - PubMed
-
- Vreys V, David G. Mammalian heparanase: what is the message? J Cell Mol Med. 2007;11:427–52. https://doi.org/10.1111/j.1582-4934.2007.00039.x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

