Detection of HIV-1 and Human Proteins in Urinary Extracellular Vesicles from HIV+ Patients
- PMID: 29721020
- PMCID: PMC5867598
- DOI: 10.1155/2018/7863412
Detection of HIV-1 and Human Proteins in Urinary Extracellular Vesicles from HIV+ Patients
Abstract
Background: Extracellular vesicles (EVs) are membrane bound, secreted by cells, and detected in bodily fluids, including urine, and contain proteins, RNA, and DNA. Our goal was to identify HIV and human proteins (HPs) in urinary EVs from HIV+ patients and compare them to HIV- samples.
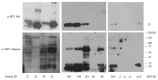
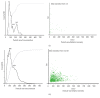
Methods: Urine samples were collected from HIV+ (n = 35) and HIV- (n = 12) individuals. EVs were isolated by ultrafiltration and characterized using transmission electron microscopy, tandem mass spectrometry (LC/MS/MS), and nanoparticle tracking analysis (NTA). Western blots confirmed the presence of HIV proteins. Gene ontology (GO) analysis was performed using FunRich and HIV Human Interaction database (HHID).
Results: EVs from urine were 30-400 nm in size. More EVs were in HIV+ patients, P < 0.05, by NTA. HIV+ samples had 14,475 HPs using LC/MS/MS, while only 111 were in HIV-. HPs in the EVs were of exosomal origin. LC/MS/MS showed all HIV+ samples contained at least one HIV protein. GO analysis showed differences in proteins between HIV+ and HIV- samples and more than 50% of the published HPs in the HHID interacted with EV HIV proteins.
Conclusion: Differences in the proteomic profile of EVs from HIV+ versus HIV- samples were found. HIV and HPs in EVs could be used to detect infection and/or diagnose HIV disease syndromes.
Figures


Similar articles
-
Proteomic analysis of cerebrospinal fluid extracellular vesicles reveals synaptic injury, inflammation, and stress response markers in HIV patients with cognitive impairment.J Neuroinflammation. 2019 Dec 5;16(1):254. doi: 10.1186/s12974-019-1617-y. J Neuroinflammation. 2019. PMID: 31805958 Free PMC article.
-
Comparing small urinary extracellular vesicle purification methods with a view to RNA sequencing-Enabling robust and non-invasive biomarker research.Biomol Detect Quantif. 2019 Jun 4;17:100089. doi: 10.1016/j.bdq.2019.100089. eCollection 2019 Mar. Biomol Detect Quantif. 2019. PMID: 31194192 Free PMC article.
-
Ultrafiltration combing with phospholipid affinity-based isolation for metabolomic profiling of urinary extracellular vesicles.J Chromatogr A. 2021 Mar 15;1640:461942. doi: 10.1016/j.chroma.2021.461942. Epub 2021 Jan 30. J Chromatogr A. 2021. PMID: 33588274
-
Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
-
Chromatography and its hyphenation to mass spectrometry for extracellular vesicle analysis.J Chromatogr A. 2016 Mar 25;1439:26-41. doi: 10.1016/j.chroma.2016.01.017. Epub 2016 Jan 11. J Chromatogr A. 2016. PMID: 26830636 Review.
Cited by
-
The role of exosomal transport of viral agents in persistent HIV pathogenesis.Retrovirology. 2018 Dec 22;15(1):79. doi: 10.1186/s12977-018-0462-x. Retrovirology. 2018. PMID: 30577804 Free PMC article. Review.
-
Latent HIV-Exosomes Induce Mitochondrial Hyperfusion Due to Loss of Phosphorylated Dynamin-Related Protein 1 in Brain Endothelium.Mol Neurobiol. 2021 Jun;58(6):2974-2989. doi: 10.1007/s12035-021-02319-8. Epub 2021 Feb 14. Mol Neurobiol. 2021. PMID: 33586027 Free PMC article.
-
Oncogenic Effects of HIV-1 Proteins, Mechanisms Behind.Cancers (Basel). 2021 Jan 15;13(2):305. doi: 10.3390/cancers13020305. Cancers (Basel). 2021. PMID: 33467638 Free PMC article. Review.
-
Extracellular Vesicles in Viral Infections of the Nervous System.Viruses. 2020 Jun 28;12(7):700. doi: 10.3390/v12070700. Viruses. 2020. PMID: 32605316 Free PMC article. Review.
-
Transcriptome-wide association study of HIV-1 acquisition identifies HERC1 as a susceptibility gene.iScience. 2022 Aug 4;25(9):104854. doi: 10.1016/j.isci.2022.104854. eCollection 2022 Sep 16. iScience. 2022. PMID: 36034232 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

