Differential anti-inflammatory effects of budesonide and a p38 MAPK inhibitor AZD7624 on COPD pulmonary cells
- PMID: 29719383
- PMCID: PMC5914546
- DOI: 10.2147/COPD.S159936
Differential anti-inflammatory effects of budesonide and a p38 MAPK inhibitor AZD7624 on COPD pulmonary cells
Abstract
Background: The effects of anti-inflammatory drugs in COPD patients may vary between different cell types. The aim of the current study was to assess the anti-inflammatory effects of the corticosteroid budesonide and a p38 MAPK inhibitor (AZD7624) on different cell types obtained from COPD patients and healthy controls.
Methods: Eight healthy smokers, 16 COPD infrequent exacerbators, and 16 frequent COPD exacerbators (≥2 exacerbations in the last year) were recruited for bronchoscopy and blood sampling. The anti-inflammatory effects of budesonide and AZD7624 were assessed on cytokine release from lipopolysaccharide-stimulated alveolar macrophages and peripheral blood mononuclear cells and polyinosinic:polycytidylic acid-stimulated bronchial epithelial cells.
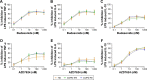
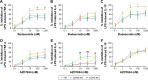
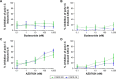
Results: The anti-inflammatory effects of budesonide varied greatly within a patient according to the cell type studied. Bronchial epithelial cells showed the lowest sensitivity to budesonide, while peripheral blood mononuclear cells showed the greatest sensitivity. AZD7624 had a greater effect than budesonide on cytokine production from bronchial epithelial cells. Exacerbation frequency did not influence corticosteroid sensitivity.
Conclusion: We observed variable corticosteroid and p38 MAPK inhibitor anti-inflammatory responses within the same individual depending on the cell type studied. These findings support the use of multiple anti-inflammatory strategies in COPD patients due to differences between cell types.
Keywords: PBMC; corticosteroid; epithelial cell; exacerbation; inflammation; macrophage.
Conflict of interest statement
Disclosure DMC and PJ are employees of AstraZeneca, which funded the study. DS has received sponsorship to attend international meetings, honoraria for lecturing or attending advisory boards, and research grants from various pharmaceutical companies including Almirall, AstraZeneca, Boehringer Ingelheim, Chiesi, Genentech, GlaxoSmithKline, Glenmark, Johnson and Johnson, Merck, NAPP, Novartis, Pfizer, Skypharma, Takeda, Teva, Therevance, and Verona. The authors report no other conflicts of interest in this work.
Figures


Similar articles
-
Role of p38 Mitogen-Activated Protein Kinase in Asthma and COPD: Pathogenic Aspects and Potential Targeted Therapies.Drug Des Devel Ther. 2021 Mar 23;15:1275-1284. doi: 10.2147/DDDT.S300988. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33790539 Free PMC article. Review.
-
The development of AZD7624 for prevention of exacerbations in COPD: a randomized controlled trial.Int J Chron Obstruct Pulmon Dis. 2018 Mar 27;13:1009-1019. doi: 10.2147/COPD.S150576. eCollection 2018. Int J Chron Obstruct Pulmon Dis. 2018. PMID: 29628759 Free PMC article. Clinical Trial.
-
Synergistic effects of p38 mitogen-activated protein kinase inhibition with a corticosteroid in alveolar macrophages from patients with chronic obstructive pulmonary disease.J Pharmacol Exp Ther. 2011 Sep;338(3):732-40. doi: 10.1124/jpet.111.180737. Epub 2011 May 24. J Pharmacol Exp Ther. 2011. PMID: 21610141
-
Reversal of corticosteroid insensitivity by p38 MAPK inhibition in peripheral blood mononuclear cells from COPD.Int J Chron Obstruct Pulmon Dis. 2015 Feb 4;10:283-91. doi: 10.2147/COPD.S72403. eCollection 2015. Int J Chron Obstruct Pulmon Dis. 2015. PMID: 25678784 Free PMC article.
-
Tristetraprolin and its role in regulation of airway inflammation.Mol Pharmacol. 2015 Apr;87(4):629-38. doi: 10.1124/mol.114.095984. Epub 2014 Nov 26. Mol Pharmacol. 2015. PMID: 25429052 Review.
Cited by
-
Role of p38 Mitogen-Activated Protein Kinase in Asthma and COPD: Pathogenic Aspects and Potential Targeted Therapies.Drug Des Devel Ther. 2021 Mar 23;15:1275-1284. doi: 10.2147/DDDT.S300988. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 33790539 Free PMC article. Review.
-
Increased mast cell activation in eosinophilic chronic obstructive pulmonary disease.Clin Transl Immunology. 2022 Sep 26;11(9):e1417. doi: 10.1002/cti2.1417. eCollection 2022. Clin Transl Immunology. 2022. PMID: 36188122 Free PMC article.
-
How inhaled corticosteroids target inflammation in COPD.Eur Respir Rev. 2023 Oct 18;32(170):230084. doi: 10.1183/16000617.0084-2023. Print 2023 Dec 31. Eur Respir Rev. 2023. PMID: 37852657 Free PMC article. Review.
-
COVID-19 and COPD: a narrative review of the basic science and clinical outcomes.Eur Respir Rev. 2020 Nov 5;29(158):200199. doi: 10.1183/16000617.0199-2020. Print 2020 Dec 31. Eur Respir Rev. 2020. PMID: 33153991 Free PMC article. Review.
-
DNA Methylation-Based Age Prediction and Telomere Length Reveal an Accelerated Aging in Induced Sputum Cells Compared to Blood Leukocytes: A Pilot Study in COPD Patients.Front Med (Lausanne). 2021 Jul 23;8:690312. doi: 10.3389/fmed.2021.690312. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34368190 Free PMC article.
References
-
- Hogg JC, Chu F, Utokaparch S, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350(26):2645–2653. - PubMed
-
- Papi A, Bellettato CM, Braccioni F, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. Am J Respir Crit Care Med. 2006;173(10):1114–1121. - PubMed
-
- Calverley P, Pauwels R, Vestbo J, et al. Combined salmeterol and fluticasone in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Lancet. 2003;361(9356):449–456. - PubMed
-
- Calverley PM, Anderson JA, Celli B, et al. Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356(8):775–789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

