Xenobiotic Nuclear Receptor Signaling Determines Molecular Pathogenesis of Progressive Familial Intrahepatic Cholestasis
- PMID: 29718219
- PMCID: PMC7263843
- DOI: 10.1210/en.2018-00110
Xenobiotic Nuclear Receptor Signaling Determines Molecular Pathogenesis of Progressive Familial Intrahepatic Cholestasis
Abstract
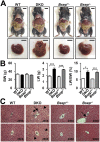
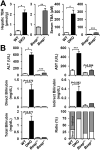
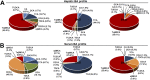
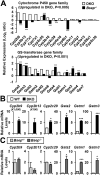
Progressive familial intrahepatic cholestasis (PFIC) is a genetically heterogeneous disorder of bile flow disruption due to abnormal canalicular transport or impaired bile acid (BA) metabolism, causing excess BA accumulation and liver failure. We previously reported an intrahepatic cholestasis mouse model based on loss of function of both farnesoid X receptor (FXR; NR1H4) and a small heterodimer partner (SHP; NR0B2) [double knockout (DKO)], which has strong similarities to human PFIC5. We compared the pathogenesis of DKO livers with that of another intrahepatic cholestasis model, Bsep-/-, which represents human PFIC2. Both models exhibit severe hepatomegaly and hepatic BA accumulation, but DKO showed greater circulating BA and liver injury, and Bsep-/- had milder phenotypes. Molecular profiling of BAs uncovered specific enrichment of cholic acid (CA)-derived BAs in DKO livers but chenodeoxycholate-derived BAs in Bsep-/- livers. Transcriptomic and proteomic analysis revealed specific activation of CA synthesis and alternative basolateral BA transport in DKO but increased chenodeoxycholic acid synthesis and canalicular transport in Bsep-/-. The constitutive androstane receptor (CAR)/pregnane X receptor (PXR)-CYP2B/CYP2C axis is activated in DKO livers but not in other cholestasis models. Loss of this axis in Fxr:Shp:Car:Pxr quadruple knockouts blocked Cyp2b/Cyp2c gene induction, impaired bilirubin conjugation/elimination, and increased liver injury. Differential CYP2B expression in DKO and Bsep-/- was recapitulated in human PFIC5 and PFIC2 livers. In conclusion, loss of FXR/SHP results in distinct molecular pathogenesis and CAR/PXR activation, which promotes Cyp2b/Cyp2c gene transcription and bilirubin clearance. CAR/PXR activation was not observed in Bsep-/- mice or PFIC2 patients. These findings provide a deeper understanding of the heterogeneity of intrahepatic cholestasis.
Figures


Similar articles
-
Constitutive Androstane Receptor Differentially Regulates Bile Acid Homeostasis in Mouse Models of Intrahepatic Cholestasis.Hepatol Commun. 2018 Dec 4;3(1):147-159. doi: 10.1002/hep4.1274. eCollection 2019 Jan. Hepatol Commun. 2018. PMID: 30620001 Free PMC article.
-
The ileum-liver Farnesoid X Receptor signaling axis mediates the compensatory mechanism of 17α-ethynylestradiol-induced cholestasis via increasing hepatic biosynthesis of chenodeoxycholic acids in rats.Eur J Pharm Sci. 2018 Oct 15;123:404-415. doi: 10.1016/j.ejps.2018.08.005. Epub 2018 Aug 2. Eur J Pharm Sci. 2018. PMID: 30077711
-
Bile salt excretory pump: biology and pathobiology.J Pediatr Gastroenterol Nutr. 2006 Jul;43 Suppl 1:S10-6. doi: 10.1097/01.mpg.0000226385.71859.5f. J Pediatr Gastroenterol Nutr. 2006. PMID: 16819395 Review.
-
Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice.Toxicol Sci. 2009 Jul;110(1):47-60. doi: 10.1093/toxsci/kfp094. Epub 2009 Apr 30. Toxicol Sci. 2009. PMID: 19407337 Free PMC article.
-
Targeting FXR in Cholestasis.Handb Exp Pharmacol. 2019;256:299-324. doi: 10.1007/164_2019_231. Handb Exp Pharmacol. 2019. PMID: 31201556 Review.
Cited by
-
Genetic alterations and molecular mechanisms underlying hereditary intrahepatic cholestasis.Front Pharmacol. 2023 May 31;14:1173542. doi: 10.3389/fphar.2023.1173542. eCollection 2023. Front Pharmacol. 2023. PMID: 37324459 Free PMC article. Review.
-
Update on FXR Biology: Promising Therapeutic Target?Int J Mol Sci. 2018 Jul 16;19(7):2069. doi: 10.3390/ijms19072069. Int J Mol Sci. 2018. PMID: 30013008 Free PMC article. Review.
-
The long and the small collide: LncRNAs and small heterodimer partner (SHP) in liver disease.Mol Cell Endocrinol. 2021 May 15;528:111262. doi: 10.1016/j.mce.2021.111262. Epub 2021 Mar 26. Mol Cell Endocrinol. 2021. PMID: 33781837 Free PMC article. Review.
-
Steatotic liver disease induced by TCPOBOP-activated hepatic constitutive androstane receptor: primary and secondary gene responses with links to disease progression.Toxicol Sci. 2024 Aug 1;200(2):324-345. doi: 10.1093/toxsci/kfae057. Toxicol Sci. 2024. PMID: 38710495
-
Transcriptomic analysis across liver diseases reveals disease-modulating activation of constitutive androstane receptor in cholestasis.JHEP Rep. 2020 Jul 2;2(5):100140. doi: 10.1016/j.jhepr.2020.100140. eCollection 2020 Oct. JHEP Rep. 2020. PMID: 32875282 Free PMC article.
References
-
- Beuers U,Trauner M,Jansen P,Poupon R. New paradigms in the treatment of hepatic cholestasis: from UDCA to FXR, PXR and beyond.J Hepatol.2015;62(1,Suppl):S25–S37. - PubMed
-
- Schady DA,Finegold MJ. Contemporary evaluation of the pediatric liver biopsy.Gastroenterol Clin North Am.2017;46(2):233–252. - PubMed
-
- Gomez-Ospina N,Potter CJ,Xiao R,Manickam K,Kim MS,Kim KH,Shneider BL,Picarsic JL,Jacobson TA,Zhang J,He W,Liu P,Knisely AS,Finegold MJ,Muzny DM,Boerwinkle E,Lupski JR,Plon SE,Gibbs RA,Eng CM,Yang Y,Washington GC,Porteus MH,Berquist WE,Kambham N,Singh RJ,Xia F,Enns GM,Moore DD. Mutations in the nuclear bile acid receptor FXR cause progressive familial intrahepatic cholestasis.Nat Commun.2016;7:10713. - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

