Comparison of the virulence of three H3N2 canine influenza virus isolates from Korea and China in mouse and Guinea pig models
- PMID: 29716608
- PMCID: PMC5930860
- DOI: 10.1186/s12917-018-1469-1
Comparison of the virulence of three H3N2 canine influenza virus isolates from Korea and China in mouse and Guinea pig models
Abstract
Background: Avian-origin H3N2 canine influenza virus (CIV) has been the most common subtype in Korea and China since 2007. Here, we compared the pathogenicity and transmissibility of three H3N2 CIV strains [Chinese CIV (JS/10), Korean CIV (KR/07), and Korean recombinant CIV between the classic H3N2 CIV and the pandemic H1N1 virus (MV/12)] in BALB/c mouse and guinea pig models. The pandemic H1N1 (CA/09) strain served as the control.
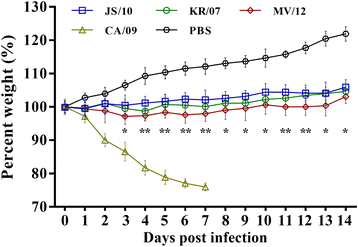
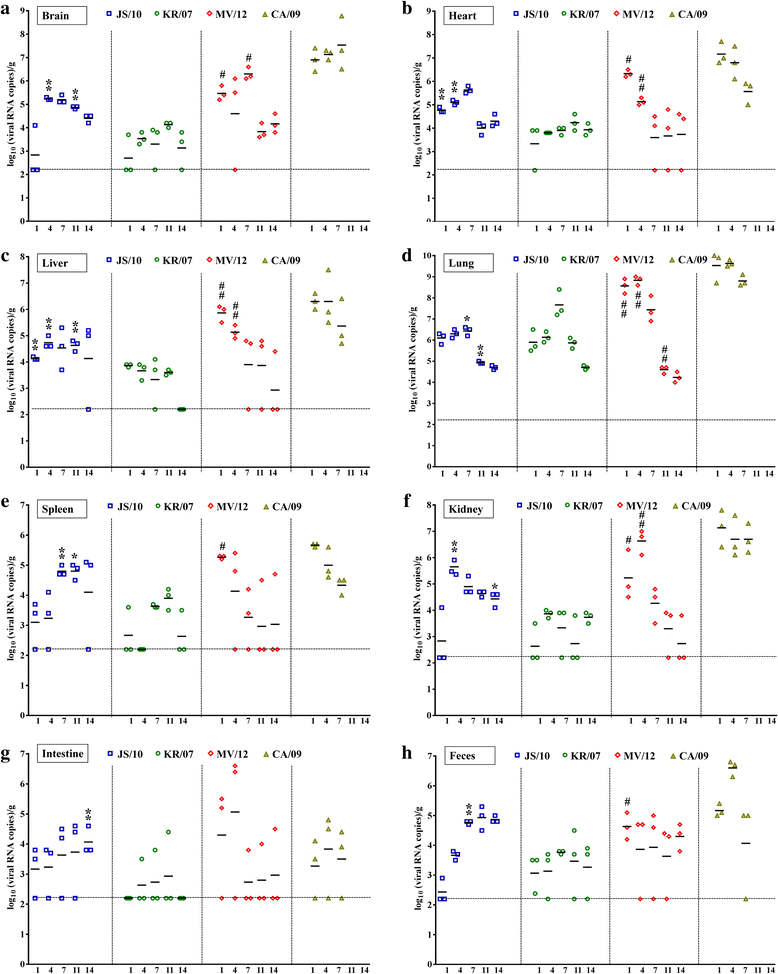
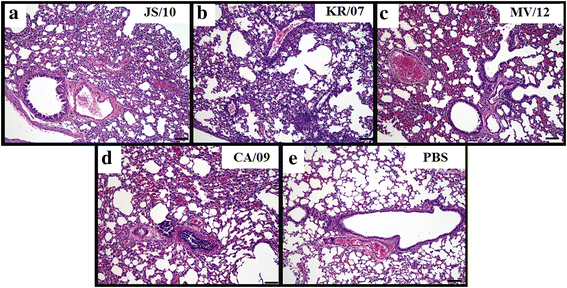
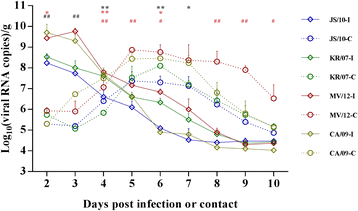
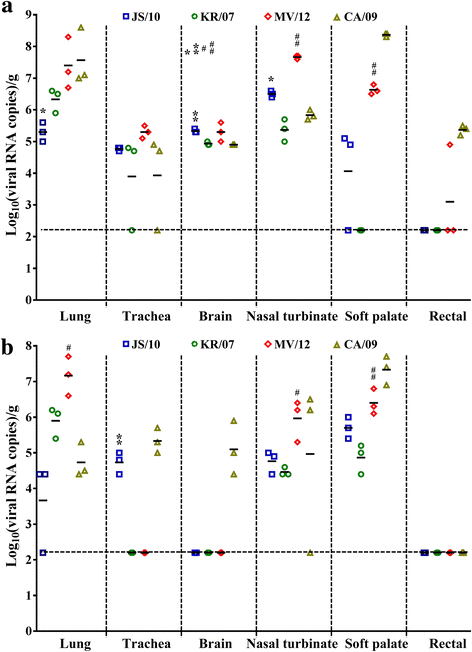
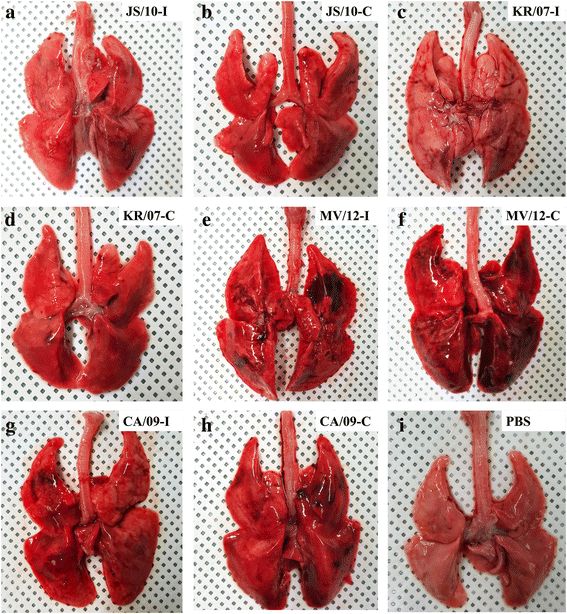
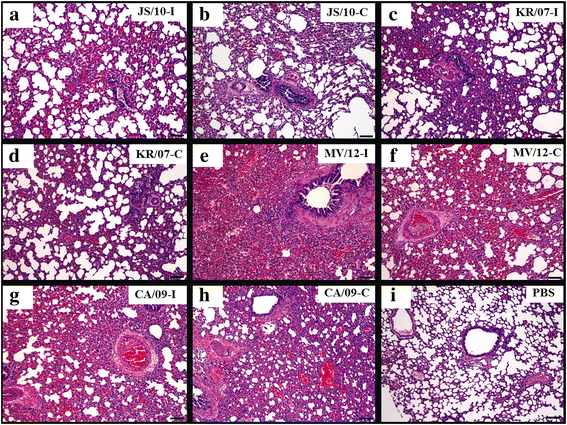
Results: BALB/c mice infected with H1N1 had high mortality and obvious body weight loss, whereas no overt disease symptoms were observed in mice inoculated with H3N2 CIV strains. The viral titers were higher in the group MV/12 than those in groups JS/10 and KR/07, while the mice infected with JS/10 showed higher viral titers in all tissues (except for the lung) than the mice infected with KR/07. The data obtained in guinea pigs also demonstrated that group MV/12 presented the highest loads in most of the tissues, followed by group JS/10 and KR/07. Also, direct contact transmissions of all the three CIV strains could be observed in guinea pigs, and for the inoculated and the contact groups, the viral titer of group MV/12 and KR/07 was higher than that of group JS/10 in nasal swabs. These findings indicated that the matrix (M) gene obtained from the pandemic H1N1 may enhance viral replication of classic H3N2 CIV; JS/10 has stronger viral replication ability in tissues as compared to KR/07, whereas KR/07 infected guinea pigs have more viral shedding than JS/10 infected guinea pigs.
Conclusions: There exists a discrepancy in pathobiology among CIV isolates. Reverse genetics regarding the genomes of CIV isolates will be helpful to further explain the virus characteristics.
Keywords: BALB/c mice; Guinea pigs; H3N2 canine influenza virus; Pathogenicity; Transmissibility.
Conflict of interest statement
Ethics approval
This study is not involved human participants. Veterinarians took the samples for analysis purposes and/or to check the health status of the mice and guinea pig population. Before conducting the study, approval for conducting the animal experiments was obtained from the Animal Ethics Committee of Korea University.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Zoonotic Risk, Pathogenesis, and Transmission of Avian-Origin H3N2 Canine Influenza Virus.J Virol. 2017 Oct 13;91(21):e00637-17. doi: 10.1128/JVI.00637-17. Print 2017 Nov 1. J Virol. 2017. PMID: 28814512 Free PMC article.
-
Viral dominance of reassortants between canine influenza H3N2 and pandemic (2009) H1N1 viruses from a naturally co-infected dog.Virol J. 2015 Sep 4;12:134. doi: 10.1186/s12985-015-0343-z. Virol J. 2015. PMID: 26336880 Free PMC article.
-
Comparative analysis of virulence of a novel, avian-origin H3N2 canine influenza virus in various host species.Virus Res. 2015 Jan 2;195:135-40. doi: 10.1016/j.virusres.2014.08.020. Epub 2014 Sep 16. Virus Res. 2015. PMID: 25218482
-
[Swine influenza virus: evolution mechanism and epidemic characterization--a review].Wei Sheng Wu Xue Bao. 2009 Sep;49(9):1138-45. Wei Sheng Wu Xue Bao. 2009. PMID: 20030049 Review. Chinese.
-
Global seroprevalence and prevalence of infection of influenza in dogs (Canis familiaris): A systematic review and meta-analysis.Rev Med Virol. 2024 May;34(3):e2542. doi: 10.1002/rmv.2542. Rev Med Virol. 2024. PMID: 38747622 Review.
Cited by
-
Role of CARD Region of MDA5 Gene in Canine Influenza Virus Infection.Viruses. 2020 Mar 12;12(3):307. doi: 10.3390/v12030307. Viruses. 2020. PMID: 32178353 Free PMC article.
-
Role of CIV NS1 Protein in Innate Immunity and Viral Replication.Int J Mol Sci. 2023 Jun 13;24(12):10056. doi: 10.3390/ijms241210056. Int J Mol Sci. 2023. PMID: 37373204 Free PMC article.
References
-
- Ali A, Daniels JB, Zhang Y, Rodriguez-Palacios A, Hayes-Ozello K, Mathes L, Lee CW. Pandemic and seasonal human influenza virus infections in domestic cats: prevalence, association with respiratory disease, and seasonality patterns. J Clin Microbiol. 2011;49:4101–4105. doi: 10.1128/JCM.05415-11. - DOI - PMC - PubMed
-
- Crawford PC, Dubovi EJ, Castleman WL, Stephenson I, Gibbs EP, Chen L, Smith C, Hill RC, Ferro P, Pompey J, Bright RA, Medina MJ, Johnson CM, Olsen CW, Cox NJ, Klimov AI, Katz JM, Donis RO. Transmission of equine influenza virus to dogs. Science. 2005;310:482–485. doi: 10.1126/science.1117950. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

