Physiologically-based pharmacokinetic model of vaginally administered dapivirine ring and film formulations
- PMID: 29714824
- PMCID: PMC6089833
- DOI: 10.1111/bcp.13625
Physiologically-based pharmacokinetic model of vaginally administered dapivirine ring and film formulations
Abstract
Aims: A physiologically-based pharmacokinetic (PBPK) model of the vaginal space was developed with the aim of predicting concentrations in the vaginal and cervical space. These predictions can be used to optimize the probability of success of vaginally administered dapivirine (DPV) for HIV prevention. We focus on vaginal delivery using either a ring or film.
Methods: A PBPK model describing the physiological structure of the vaginal tissue and fluid was defined mathematically and implemented in MATLAB. Literature reviews provided estimates for relevant physiological and physiochemical parameters. Drug concentration-time profiles were simulated in luminal fluids, vaginal tissue and plasma after administration of ring or film. Patient data were extracted from published clinical trials and used to test model predictions.
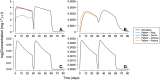
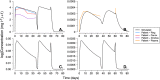
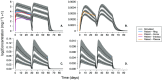
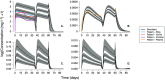
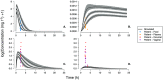
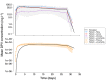
Results: The DPV ring simulations tested the two dosing regimens and predicted PK profiles and area under the curve of luminal fluids (29 079 and 33 067 mg h l-1 in groups A and B, respectively) and plasma (0.177 and 0.211 mg h l-1 ) closely matched those reported (within one standard deviation). While the DPV film study reported drug concentration at only one time point per patient, our simulated profiles pass through reported concentration range.
Conclusions: HIV is a major public health issue and vaginal microbicides have the potential to provide a crucial, female-controlled option for protection. The PBPK model successfully simulated realistic representations of drug PK. It provides a reliable, inexpensive and accessible platform where potential effectiveness of new compounds and the robustness of treatment modalities for pre-exposure prophylaxis can be evaluated.
Keywords: antiretrovirals; pharmacokinetics; pharmacometrics.
© 2018 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Figures


Similar articles
-
The sheep as a model of preclinical safety and pharmacokinetic evaluations of candidate microbicides.Antimicrob Agents Chemother. 2015 Jul;59(7):3761-70. doi: 10.1128/AAC.04954-14. Epub 2015 Apr 6. Antimicrob Agents Chemother. 2015. PMID: 25845860 Free PMC article.
-
Comparison of Dapivirine Vaginal Gel and Film Formulation Pharmacokinetics and Pharmacodynamics (FAME 02B).AIDS Res Hum Retroviruses. 2017 Apr;33(4):339-346. doi: 10.1089/AID.2016.0040. Epub 2016 Dec 13. AIDS Res Hum Retroviruses. 2017. PMID: 27809557 Free PMC article.
-
Development and verification of mechanistic vaginal absorption and metabolism model to predict systemic exposure after vaginal ring and gel application.Br J Clin Pharmacol. 2024 Jun;90(6):1428-1449. doi: 10.1111/bcp.16029. Epub 2024 Mar 7. Br J Clin Pharmacol. 2024. PMID: 38450818
-
Development of dapivirine vaginal ring for HIV prevention.Antiviral Res. 2013 Dec;100 Suppl:S3-8. doi: 10.1016/j.antiviral.2013.09.025. Epub 2013 Nov 1. Antiviral Res. 2013. PMID: 24188702 Review.
-
Vaginal drug distribution modeling.Adv Drug Deliv Rev. 2015 Sep 15;92:2-13. doi: 10.1016/j.addr.2015.04.017. Epub 2015 Apr 28. Adv Drug Deliv Rev. 2015. PMID: 25933938 Free PMC article. Review.
Cited by
-
Modeling HIV Pre-Exposure Prophylaxis.Front Pharmacol. 2020 Jan 31;10:1514. doi: 10.3389/fphar.2019.01514. eCollection 2019. Front Pharmacol. 2020. PMID: 32082142 Free PMC article. Review.
-
Transport and Permeation Properties of Dapivirine: Understanding Potential Drug-Drug Interactions.Pharmaceutics. 2022 Sep 14;14(9):1948. doi: 10.3390/pharmaceutics14091948. Pharmaceutics. 2022. PMID: 36145696 Free PMC article.
-
Building in-house PBPK modelling tools for oral drug administration from literature information.ADMET DMPK. 2019 Feb 23;7(1):4-21. doi: 10.5599/admet.638. eCollection 2019. ADMET DMPK. 2019. PMID: 35350741 Free PMC article.
-
Mechanistic modeling of ophthalmic, nasal, injectable, and implant generic drug products: A workshop summary report.CPT Pharmacometrics Syst Pharmacol. 2023 May;12(5):631-638. doi: 10.1002/psp4.12952. Epub 2023 Mar 20. CPT Pharmacometrics Syst Pharmacol. 2023. PMID: 36851886 Free PMC article. Review.
-
The Promise of Long-Acting Antiretroviral Therapies: From Need to Manufacture.Trends Microbiol. 2019 Jul;27(7):593-606. doi: 10.1016/j.tim.2019.02.009. Epub 2019 Apr 10. Trends Microbiol. 2019. PMID: 30981593 Free PMC article. Review.
References
-
- UNAIDS . Fact sheet: latest statistics on the status of the AIDS epidemic 2016. Available at http://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet_e... (last accessed 10 April 2017).
-
- World Health Organization . Global health sector response to HIV, 2000‐2015. 2016: 1–109.
-
- World Health Organization . Guideline on when to start antiretroviral therapy and on pre‐exposure prophylaxis for HIV. 2015: 1–76. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

