Identification of a novel Dlg2 isoform differentially expressed in IFNβ-producing plasmacytoid dendritic cells
- PMID: 29703139
- PMCID: PMC6389146
- DOI: 10.1186/s12864-018-4573-5
Identification of a novel Dlg2 isoform differentially expressed in IFNβ-producing plasmacytoid dendritic cells
Abstract
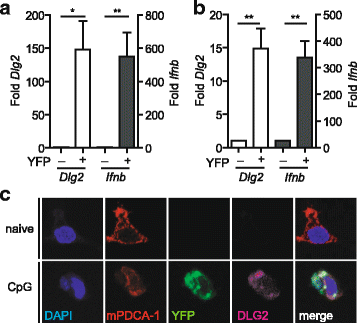
Background: The murine discs large homolog 2 (DLG2; post synaptic density 93 (PSD-93); Chapsyn-110) is a member of the membrane-associated guanylate kinase (MAGUK) protein family involved in receptor assembly and associated with signaling enzymes on cell membranes. In neurons, DLG2 protein isoforms derived from alternatively spliced transcripts have been described to bind to NMDA (N-methyl-aspartate) receptors and K channels and to mediate clustering of these channels in the postsynaptic membrane. In myeloid cells of the immune system, such as dendritic cells (DCs), a lack of data exists on the expression or function of DLG2. In cDNA microarray transcriptome analyses, we found Dlg2 highly expressed in a subpopulation of plasmacytoid DCs (pDCs) stimulated to produce type I interferons (IFNs) such as IFNβ.
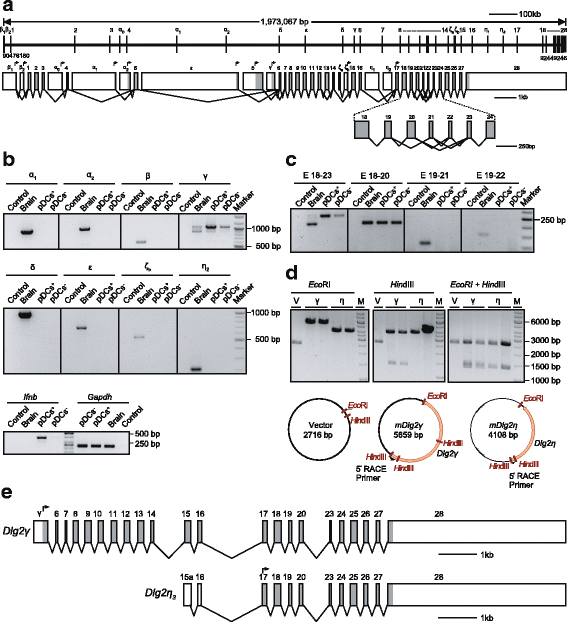
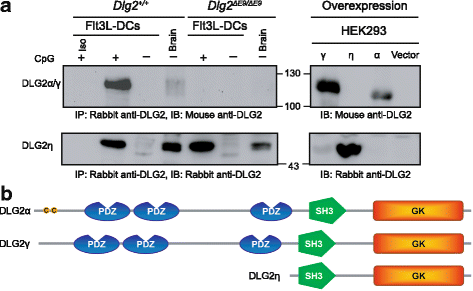
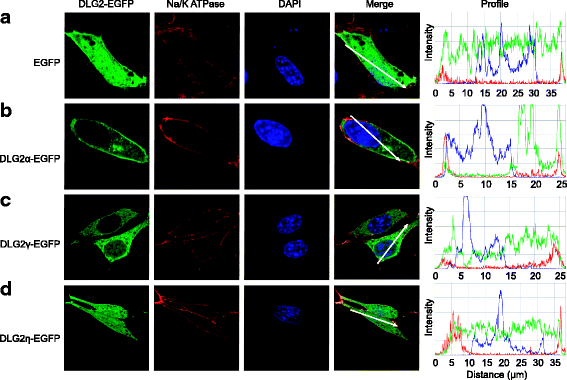
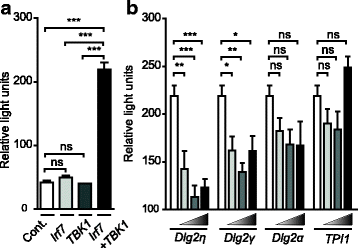
Results: Using RACE- and RT-PCR as well as immunoprecipitation followed by Western blotting we characterised the differential expression of the Dlg2 splice variants in IFNβ-producing pDCs. Besides Dlg2ɣ this cell population expressed a novel short Dlg2η transcript we termed Dlg2η3. Our expression data were integrated into information from genome databases to obtain a novel and comprehensive overview of the mouse Dlg2 gene architecture. To elucidate the intracellular localisation pattern of protein isoforms, ectopical expression analysis of fluorescently tagged DLG2 splice variants was performed. Here we found an enrichment of the larger isoform DLG2α1 at the plasma membrane while the newly identified shorter (DLG2η) isoform as well as DLG2ɣ were equally distributed throughout the cytoplasm. Additionally, DLG2η was also found in the nucleus. Analysis of Dlg2-knockout mice previously generated by deleting exon 9 surprisingly revealed that the protein for the novel DLG2η isoform was still expressed in the brain and in bone marrow-derived pDCs from mice carrying the homozygous deletion (Dlg2 ΔE9/ΔE9 ).
Conclusion: We describe a novel splice variant of the mouse Dlg2 gene termed Dlg2η and define the differential expression pattern of DLG2 isoforms in IFNβ-producing pDCs. The presence of DLG2η protein in the CNS of Dlg2 ΔE9/ΔE9 mice might influence the phenotype of these mice and has to be taken into account in the interpretation of results regarding the functional role of DLG2 in neuronal postsynaptic membranes.
Keywords: Dlg2; IFNβ; Isoforms; PSD-93; Plasmacytoid dendritic cells.
Conflict of interest statement
Ethics approval and consent to participate
This study was carried out in strict accordance with the German act for animal welfare (Tierschutzgesetz) §8. The protocol was approved by the local ethics committee, the board for nature, environment and consumer protection (Landesamt für Natur-, Umwelt-, und Verbraucherschutz – LANUV) of the regional government of Düsseldorf (North Rhine-Westphalia, Germany); Permit number 84–02.05.30.13.034. All efforts were made to minimize suffering.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
A DLG2 deficiency in mice leads to reduced sociability and increased repetitive behavior accompanied by aberrant synaptic transmission in the dorsal striatum.Mol Autism. 2020 Mar 12;11(1):19. doi: 10.1186/s13229-020-00324-7. Mol Autism. 2020. PMID: 32164788 Free PMC article.
-
DlgS97/SAP97, a neuronal isoform of discs large, regulates ethanol tolerance.PLoS One. 2012;7(11):e48967. doi: 10.1371/journal.pone.0048967. Epub 2012 Nov 7. PLoS One. 2012. PMID: 23145041 Free PMC article.
-
PTBP-dependent PSD-95 and CamKIIα alternative splicing in the lens.Mol Vis. 2014 Dec 12;20:1660-7. eCollection 2014. Mol Vis. 2014. PMID: 25540577 Free PMC article.
-
Differential roles of postsynaptic density-93 isoforms in regulating synaptic transmission.J Neurosci. 2013 Sep 25;33(39):15504-17. doi: 10.1523/JNEUROSCI.0019-12.2013. J Neurosci. 2013. PMID: 24068818 Free PMC article.
-
The anchoring protein SAP97 influences the trafficking and localisation of multiple membrane channels.Biochim Biophys Acta. 2014 Feb;1838(2):589-94. doi: 10.1016/j.bbamem.2013.03.015. Epub 2013 Mar 25. Biochim Biophys Acta. 2014. PMID: 23535319 Review.
Cited by
-
Exploring the Impact of Cerebrovascular Disease and Major Depression on Non-diseased Human Tissue Transcriptomes.Front Genet. 2021 Jul 19;12:696836. doi: 10.3389/fgene.2021.696836. eCollection 2021. Front Genet. 2021. PMID: 34349785 Free PMC article.
-
MicroRNA-23a depletion promotes apoptosis of ovarian cancer stem cell and inhibits cell migration by targeting DLG2.Cancer Biol Ther. 2019;20(6):897-911. doi: 10.1080/15384047.2019.1579960. Epub 2019 Mar 12. Cancer Biol Ther. 2019. PMID: 30862230 Free PMC article.
-
Genetic alterations in the neuronal development genes are associated with changes of the tumor immune microenvironment in pancreatic cancer.Ann Pancreat Cancer. 2023 Nov;6(10):10.21037/apc-23-13. doi: 10.21037/apc-23-13. Epub 2023 Nov 20. Ann Pancreat Cancer. 2023. PMID: 38495381 Free PMC article.
-
Identifying novel genetic loci associated with polycystic ovary syndrome based on its shared genetic architecture with type 2 diabetes.Front Genet. 2022 Aug 29;13:905716. doi: 10.3389/fgene.2022.905716. eCollection 2022. Front Genet. 2022. PMID: 36105080 Free PMC article.
-
Differential Expression of Mitogen-Activated Protein Kinase Signaling Pathways in the Human Choroid-Retinal Pigment Epithelial Complex Indicates Regional Predisposition to Disease.Int J Mol Sci. 2024 Sep 20;25(18):10105. doi: 10.3390/ijms251810105. Int J Mol Sci. 2024. PMID: 39337590 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- Research Commission of the Medical Faculty 30/2016/Heinrich-Heine-Universität Düsseldorf
- SCHE692/3-1/Deutsche Forschungsgemeinschaft
- SCHE692/4-1/Deutsche Forschungsgemeinschaft
- EXC 1003, Grant FF-2014-01 Cells in Motion-Cluster of Excellence/Deutsche Forschungsgemeinschaft
- IZKF, Grant Alf3/018/16/Deutsche Forschungsgemeinschaft
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

