Characterization of HIV-1 gp120 antibody specificities induced in anogenital secretions of RV144 vaccine recipients after late boost immunizations
- PMID: 29702672
- PMCID: PMC5922559
- DOI: 10.1371/journal.pone.0196397
Characterization of HIV-1 gp120 antibody specificities induced in anogenital secretions of RV144 vaccine recipients after late boost immunizations
Abstract
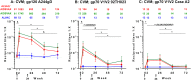
Sexual transmission is the principal driver of the human immunodeficiency virus (HIV) pandemic. Understanding HIV vaccine-induced immune responses at mucosal surfaces can generate hypotheses regarding mechanisms of protection, and may influence vaccine development. The RV144 (ClinicalTrials.gov NCT00223080) efficacy trial showed protection against HIV infections but mucosal samples were not collected, therefore, the contribution of mucosal antibodies to preventing HIV-1 acquisition is unknown. Here, we report the generation, magnitude and persistence of antibody responses to recombinant gp120 envelope and antigens including variable one and two loop scaffold antigens (gp70V1V2) previously shown to correlate with risk in RV144. We evaluated antibody responses to gp120 A244gD and gp70V1V2 92TH023 (both CRF01_AE) and Case A2 (subtype B) in cervico-vaginal mucus (CVM), seminal plasma (SP) and rectal secretions (RS) from HIV-uninfected RV144 vaccine recipients, who were randomized to receive two late boosts of ALVAC-HIV/AIDSVAX®B/E, AIDSVAX®B/E, or ALVAC-HIV alone at 0 and 6 months. Late vaccine boosting increased IgG geometric mean titers (GMT) to gp120 A244gD in AIDSVAX®B/E and ALVAC-HIV/AIDSVAX®B/E CVM (28 and 17 fold, respectively), followed by SP and RS. IgG to gp70V1V2 92TH023 increased in AIDSVAX®B/E and ALVAC-HIV/AIDSVAX®B/E CVM (11-17 fold) and SP (2 fold) two weeks post first boost. IgG to Case A2 was only detected in AIDSVAX®B/E and ALVAC-HIV/AIDSVAX®B/E CVM. Mucosal IgG to gp120 A244gD (CVM, SP, RS), gp70V1V2 92TH023 (CVM, SP), and Case A2 (CVM) correlated with plasma IgG levels (p<0.001). Although the magnitude of IgG responses declined after boosting, anti-gp120 A244gD IgG responses in CVM persisted for 12 months post final vaccination. Further studies in localization, persistence and magnitude of envelope specific antibodies (IgG and dimeric IgA) in anogenital secretions will help determine their role in preventing mucosal HIV acquisition.
Conflict of interest statement
Figures



Similar articles
-
Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial.Lancet HIV. 2020 Apr;7(4):e238-e248. doi: 10.1016/S2352-3018(19)30406-0. Epub 2020 Feb 6. Lancet HIV. 2020. PMID: 32035516 Free PMC article. Clinical Trial.
-
Randomized, Double-Blind Evaluation of Late Boost Strategies for HIV-Uninfected Vaccine Recipients in the RV144 HIV Vaccine Efficacy Trial.J Infect Dis. 2017 Apr 15;215(8):1255-1263. doi: 10.1093/infdis/jix099. J Infect Dis. 2017. PMID: 28329190 Free PMC article. Clinical Trial.
-
IgG Antibody Responses to Recombinant gp120 Proteins, gp70V1/V2 Scaffolds, and a CyclicV2 Peptide in Thai Phase I/II Vaccine Trials Using Different Vaccine Regimens.AIDS Res Hum Retroviruses. 2015 Nov;31(11):1178-86. doi: 10.1089/AID.2015.0034. Epub 2015 Sep 9. AIDS Res Hum Retroviruses. 2015. PMID: 26234467 Free PMC article. Clinical Trial.
-
Lessons from the RV144 Thai phase III HIV-1 vaccine trial and the search for correlates of protection.Annu Rev Med. 2015;66:423-37. doi: 10.1146/annurev-med-052912-123749. Epub 2014 Oct 17. Annu Rev Med. 2015. PMID: 25341006 Review.
-
Nonneutralizing functional antibodies: a new "old" paradigm for HIV vaccines.Clin Vaccine Immunol. 2014 Aug;21(8):1023-36. doi: 10.1128/CVI.00230-14. Epub 2014 Jun 11. Clin Vaccine Immunol. 2014. PMID: 24920599 Free PMC article. Review.
Cited by
-
Reverse development of vaccines against antimicrobial-resistant pathogens.NPJ Vaccines. 2024 Apr 3;9(1):71. doi: 10.1038/s41541-024-00858-4. NPJ Vaccines. 2024. PMID: 38570502 Free PMC article. Review.
-
Peptides for Vaccine Development.ACS Appl Bio Mater. 2022 Mar 21;5(3):905-944. doi: 10.1021/acsabm.1c01238. Epub 2022 Feb 23. ACS Appl Bio Mater. 2022. PMID: 35195008 Free PMC article. Review.
-
Late boosting of the RV144 regimen with AIDSVAX B/E and ALVAC-HIV in HIV-uninfected Thai volunteers: a double-blind, randomised controlled trial.Lancet HIV. 2020 Apr;7(4):e238-e248. doi: 10.1016/S2352-3018(19)30406-0. Epub 2020 Feb 6. Lancet HIV. 2020. PMID: 32035516 Free PMC article. Clinical Trial.
-
Effects of gp120 Inner Domain (ID2) Immunogen Doses on Elicitation of Anti-HIV-1 Functional Fc-Effector Response to C1/C2 (Cluster A) Epitopes in Mice.Microorganisms. 2020 Sep 28;8(10):1490. doi: 10.3390/microorganisms8101490. Microorganisms. 2020. PMID: 32998443 Free PMC article.
-
Peptide-Based Vaccines: Current Progress and Future Challenges.Chem Rev. 2020 Mar 25;120(6):3210-3229. doi: 10.1021/acs.chemrev.9b00472. Epub 2019 Dec 5. Chem Rev. 2020. PMID: 31804810 Free PMC article. Review.
References
-
- Fauci AS, Marston HD. Ending AIDS—Is an HIV Vaccine Necessary? N Engl J Med. 2014;370(6):495–8. doi: 10.1056/NEJMp1313771 - DOI - PubMed
-
- Kaul R, Pettengell C, Sheth PM, Sunderji S, Biringer A, MacDonald K, et al. The genital tract immune milieu: an important determinant of HIV susceptibility and secondary transmission. J Reprod Immunol. 2008;77(1):32–40. doi: 10.1016/j.jri.2007.02.002 - DOI - PubMed
-
- Ghosh M, Fahey JV, Shen Z, Lahey T, Cu-Uvin S, Wu Z, et al. Anti-HIV Activity in Cervical-Vaginal Secretions from HIV-Positive and -Negative Women Correlate with Innate Antimicrobial Levels and IgG Antibodies. PLoS ONE. 2010;5(6):e11366 doi: 10.1371/journal.pone.0011366 - DOI - PMC - PubMed
-
- Stax MJ, van Montfort T, Sprenger RR, Melchers M, Sanders RW, van Leeuwen E, et al. Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4+ T-lymphocytes. Virology. 2009;391(2):203–11. doi: 10.1016/j.virol.2009.06.011 - DOI - PubMed
-
- Devito C, Broliden K, Kaul R, Svensson L, Johansen K, Kiama P, et al. Mucosal and Plasma IgA from HIV-1-Exposed Uninfected Individuals Inhibit HIV-1 Transcytosis Across Human Epithelial Cells. J Immunol. 2000;165(9):5170–6. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

