Novel activities of safe-in-human broad-spectrum antiviral agents
- PMID: 29698664
- PMCID: PMC7113852
- DOI: 10.1016/j.antiviral.2018.04.016
Novel activities of safe-in-human broad-spectrum antiviral agents
Abstract
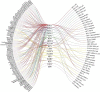
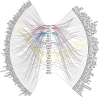
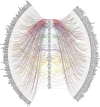
According to the WHO, there is an urgent need for better control of viral diseases. Re-positioning existing safe-in-human antiviral agents from one viral disease to another could play a pivotal role in this process. Here, we reviewed all approved, investigational and experimental antiviral agents, which are safe in man, and identified 59 compounds that target at least three viral diseases. We tested 55 of these compounds against eight different RNA and DNA viruses. We found novel activities for dalbavancin against echovirus 1, ezetimibe against human immunodeficiency virus 1 and Zika virus, as well as azacitidine, cyclosporine, minocycline, oritavancin and ritonavir against Rift valley fever virus. Thus, the spectrum of antiviral activities of existing antiviral agents could be expanded towards other viral diseases.
Copyright © 2018 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine.Viruses. 2019 Oct 18;11(10):964. doi: 10.3390/v11100964. Viruses. 2019. PMID: 31635418 Free PMC article.
-
Curcumin as an Antiviral Agent.Viruses. 2020 Oct 31;12(11):1242. doi: 10.3390/v12111242. Viruses. 2020. PMID: 33142686 Free PMC article. Review.
-
Beyond malaria: The inhibition of viruses by artemisinin-type compounds.Biotechnol Adv. 2018 Nov 1;36(6):1730-1737. doi: 10.1016/j.biotechadv.2018.01.001. Epub 2018 Jan 3. Biotechnol Adv. 2018. PMID: 29305894 Review. No abstract available.
-
Antiviral properties of non-nucleosidal alkylated pyrimidines against DNA and RNA viruses.Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968;259(2):169-72. doi: 10.1007/BF00537763. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1968. PMID: 4232662 No abstract available.
-
Antiviral action of rifampin.N Engl J Med. 1971 Mar 25;284(12):675. doi: 10.1056/NEJM197103252841219. N Engl J Med. 1971. PMID: 5545617 No abstract available.
Cited by
-
New Approaches to the Prevention and Treatment of Viral Diseases.Arch Immunol Ther Exp (Warsz). 2021 Apr 3;69(1):10. doi: 10.1007/s00005-021-00613-w. Arch Immunol Ther Exp (Warsz). 2021. PMID: 33811524 Free PMC article. Review.
-
Cyclosporin A: A Repurposable Drug in the Treatment of COVID-19?Front Med (Lausanne). 2021 Sep 6;8:663708. doi: 10.3389/fmed.2021.663708. eCollection 2021. Front Med (Lausanne). 2021. PMID: 34552938 Free PMC article. Review.
-
Identification and Tracking of Antiviral Drug Combinations.Viruses. 2020 Oct 18;12(10):1178. doi: 10.3390/v12101178. Viruses. 2020. PMID: 33080984 Free PMC article.
-
Novel Synergistic Anti-Enteroviral Drug Combinations.Viruses. 2022 Aug 25;14(9):1866. doi: 10.3390/v14091866. Viruses. 2022. PMID: 36146673 Free PMC article.
-
Selected nucleos(t)ide-based prescribed drugs and their multi-target activity.Eur J Pharmacol. 2019 Dec 15;865:172747. doi: 10.1016/j.ejphar.2019.172747. Epub 2019 Oct 18. Eur J Pharmacol. 2019. PMID: 31634460 Free PMC article. Review.
References
-
- Andrei G., Topalis D., De Schutter T., Snoeck R. Insights into the mechanism of action of cidofovir and other acyclic nucleoside phosphonates against polyoma- and papillomaviruses and non-viral induced neoplasia. Antivir. Res. 2015;114:21–46. - PubMed
-
- Barrows N.J., Campos R.K., Powell S.T., Prasanth K.R., Schott-Lerner G., Soto-Acosta R., Galarza-Munoz G., McGrath E.L., Urrabaz-Garza R., Gao J., Wu P., Menon R., Saade G., Fernandez-Salas I., Rossi S.L., Vasilakis N., Routh A., Bradrick S.S., Garcia-Blanco M.A. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe. 2016;20:259–270. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

