Bronchial mucosal IFN-α/β and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations
- PMID: 29698627
- PMCID: PMC6320262
- DOI: 10.1016/j.jaci.2018.04.003
Bronchial mucosal IFN-α/β and pattern recognition receptor expression in patients with experimental rhinovirus-induced asthma exacerbations
Abstract
Background: The innate immune system senses viral infection through pattern recognition receptors (PRRs), leading to type I interferon production. The role of type I interferon and PPRs in rhinovirus-induced asthma exacerbations in vivo are uncertain.
Objectives: We sought to compare bronchial mucosal type I interferon and PRR expression at baseline and after rhinovirus infection in atopic asthmatic patients and control subjects.
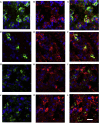
Methods: Immunohistochemistry was used to detect expression of IFN-α, IFN-β, and the PRRs: Toll-like receptor 3, melanoma differentiation-associated gene 5, and retinoic acid-inducible protein I in bronchial biopsy specimens from 10 atopic asthmatic patients and 15 nonasthmatic nonatopic control subjects at baseline and on day 4 and 6 weeks after rhinovirus infection.
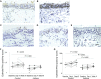
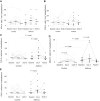
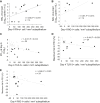
Results: We observed IFN-α/β deficiency in the bronchial epithelium at 3 time points in asthmatic patients in vivo. Lower epithelial IFN-α/β expression was related to greater viral load, worse airway symptoms, airway hyperresponsiveness, and reductions in lung function during rhinovirus infection. We found lower frequencies of bronchial subepithelial monocytes/macrophages expressing IFN-α/β in asthmatic patients during infection. Interferon deficiency at baseline was not accompanied by deficient PRR expression in asthmatic patients. Both epithelial and subepithelial PRR expression were induced during rhinovirus infection. Rhinovirus infection-increased numbers of subepithelial interferon/PRR-expressing inflammatory cells were related to greater viral load, airway hyperresponsiveness, and reductions in lung function.
Conclusions: Bronchial epithelial IFN-α/β expression and numbers of subepithelial IFN-α/β-expressing monocytes/macrophages during infection were both deficient in asthmatic patients. Lower epithelial IFN-α/β expression was associated with adverse clinical outcomes after rhinovirus infection in vivo. Increases in numbers of subepithelial cells expressing interferon/PRRs during infection were also related to greater viral load/illness severity.
Keywords: Asthma exacerbation; pattern recognition receptors; rhinovirus infection; type I interferon.
Copyright © 2018 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Similar articles
-
Deficient antiviral immune responses in childhood: distinct roles of atopy and asthma.J Allergy Clin Immunol. 2012 Dec;130(6):1307-14. doi: 10.1016/j.jaci.2012.08.005. Epub 2012 Sep 13. J Allergy Clin Immunol. 2012. PMID: 22981791
-
Rhinovirus 16-induced IFN-α and IFN-β are deficient in bronchoalveolar lavage cells in asthmatic patients.J Allergy Clin Immunol. 2012 Jun;129(6):1506-1514.e6. doi: 10.1016/j.jaci.2012.03.044. J Allergy Clin Immunol. 2012. PMID: 22657407
-
TLR3 and MDA5 signalling, although not expression, is impaired in asthmatic epithelial cells in response to rhinovirus infection.Clin Exp Allergy. 2014 Jan;44(1):91-101. doi: 10.1111/cea.12218. Clin Exp Allergy. 2014. PMID: 24131248
-
Regulation and Function of Interferon-Lambda (IFNλ) and Its Receptor in Asthma.Front Immunol. 2021 Nov 10;12:731807. doi: 10.3389/fimmu.2021.731807. eCollection 2021. Front Immunol. 2021. PMID: 34899691 Free PMC article. Review.
-
Innate Immune Responses by Respiratory Viruses, Including Rhinovirus, During Asthma Exacerbation.Front Immunol. 2022 Jun 20;13:865973. doi: 10.3389/fimmu.2022.865973. eCollection 2022. Front Immunol. 2022. PMID: 35795686 Free PMC article. Review.
Cited by
-
Mechanism of Rhinovirus Immunity and Asthma.Front Immunol. 2021 Oct 6;12:731846. doi: 10.3389/fimmu.2021.731846. eCollection 2021. Front Immunol. 2021. PMID: 34691038 Free PMC article. Review.
-
Rhinovirus-induced epithelial RIG-I inflammasome suppresses antiviral immunity and promotes inflammation in asthma and COVID-19.Nat Commun. 2023 Apr 22;14(1):2329. doi: 10.1038/s41467-023-37470-4. Nat Commun. 2023. PMID: 37087523 Free PMC article.
-
Viruses and asthma: the role of common respiratory viruses in asthma and its potential meaning for SARS-CoV-2.Immunology. 2020 Oct;161(2):83-93. doi: 10.1111/imm.13240. Epub 2020 Aug 17. Immunology. 2020. PMID: 32687609 Free PMC article. Review.
-
Innate Immune Responses and Pulmonary Diseases.Adv Exp Med Biol. 2021;1304:53-71. doi: 10.1007/978-3-030-68748-9_4. Adv Exp Med Biol. 2021. PMID: 34019263
-
Altered cell function and increased replication of rhinoviruses and EV-D68 in airway epithelia of asthma patients.Front Microbiol. 2023 Mar 1;14:1106945. doi: 10.3389/fmicb.2023.1106945. eCollection 2023. Front Microbiol. 2023. PMID: 36937308 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

