Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study
- PMID: 29695594
- PMCID: PMC5957306
- DOI: 10.1212/WNL.0000000000005516
Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study
Erratum in
-
Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study.Neurology. 2018 Sep 11;91(11):538. doi: 10.1212/WNL.0000000000005929. Neurology. 2018. PMID: 30201753 No abstract available.
Abstract
Objective: To assess dose-response effects of the anti-CD20 monoclonal antibody ofatumumab on efficacy and safety outcomes in a phase 2b double-blind study of relapsing forms of multiple sclerosis (RMS).
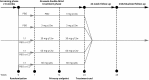
Methods: Patients (n = 232) were randomized to ofatumumab 3, 30, or 60 mg every 12 weeks, ofatumumab 60 mg every 4 weeks, or placebo for a 24-week treatment period, with a primary endpoint of cumulative number of new gadolinium-enhancing lesions (per brain MRI) at week 12. Relapses and safety/tolerability were assessed, and CD19+ peripheral blood B-lymphocyte counts measured. Safety monitoring continued weeks 24 to 48 with subsequent individualized follow-up evaluating B-cell repletion.
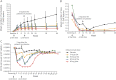
Results: The cumulative number of new lesions was reduced by 65% for all ofatumumab dose groups vs placebo (p < 0.001). Post hoc analysis (excluding weeks 1-4) estimated a ≥90% lesion reduction vs placebo (week 12) for all cumulative ofatumumab doses ≥30 mg/12 wk. Dose-dependent CD19 B-cell depletion was observed. Notably, complete depletion was not necessary for a robust treatment effect. The most common adverse event was injection-related reactions (52% ofatumumab, 15% placebo), mild to moderate severity in 97%, most commonly associated with the first dose and diminishing on subsequent dosing.
Conclusion: Imaging showed that all subcutaneous ofatumumab doses demonstrated efficacy (most robust: cumulative doses ≥30 mg/12 wk), with a safety profile consistent with existing ofatumumab data. This treatment effect also occurred with dosage regimens that only partially depleted circulating B cells.
Classification of evidence: This study provides Class I evidence that for patients with RMS, ofatumumab decreases the number of new MRI gadolinium-enhancing lesions 12 weeks after treatment initiation.
Trial registration: ClinicalTrials.gov NCT01457924.
© 2018 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
Comment in
-
Reader response: Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study.Neurology. 2019 Mar 12;92(11):542-543. doi: 10.1212/WNL.0000000000007084. Neurology. 2019. PMID: 30858244 No abstract available.
-
Author response: Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: The MIRROR study.Neurology. 2019 Mar 12;92(11):543. doi: 10.1212/WNL.0000000000007085. Neurology. 2019. PMID: 30858245 No abstract available.
Similar articles
-
Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study.Neurology. 2014 Feb 18;82(7):573-81. doi: 10.1212/WNL.0000000000000125. Epub 2014 Jan 22. Neurology. 2014. PMID: 24453078 Clinical Trial.
-
Ofatumumab versus Teriflunomide in Multiple Sclerosis.N Engl J Med. 2020 Aug 6;383(6):546-557. doi: 10.1056/NEJMoa1917246. N Engl J Med. 2020. PMID: 32757523 Clinical Trial.
-
Safety and efficacy of tolebrutinib, an oral brain-penetrant BTK inhibitor, in relapsing multiple sclerosis: a phase 2b, randomised, double-blind, placebo-controlled trial.Lancet Neurol. 2021 Sep;20(9):729-738. doi: 10.1016/S1474-4422(21)00237-4. Lancet Neurol. 2021. PMID: 34418400 Free PMC article. Clinical Trial.
-
Mitoxantrone: a review of its use in multiple sclerosis.CNS Drugs. 2004;18(6):379-96. doi: 10.2165/00023210-200418060-00010. CNS Drugs. 2004. PMID: 15089110 Review.
-
The Development of Ofatumumab, a Fully Human Anti-CD20 Monoclonal Antibody for Practical Use in Relapsing Multiple Sclerosis Treatment.Neurol Ther. 2023 Oct;12(5):1491-1515. doi: 10.1007/s40120-023-00518-0. Epub 2023 Jul 14. Neurol Ther. 2023. PMID: 37450172 Free PMC article. Review.
Cited by
-
Inhibition of Bruton's tyrosine kinase interferes with pathogenic B-cell development in inflammatory CNS demyelinating disease.Acta Neuropathol. 2020 Oct;140(4):535-548. doi: 10.1007/s00401-020-02204-z. Epub 2020 Aug 6. Acta Neuropathol. 2020. PMID: 32761407 Free PMC article.
-
Personalized Use of Disease-Modifying Therapies in Multiple Sclerosis.Pharmaceutics. 2024 Jan 17;16(1):120. doi: 10.3390/pharmaceutics16010120. Pharmaceutics. 2024. PMID: 38258130 Free PMC article. Review.
-
Role of B Cells in Multiple Sclerosis and Related Disorders.Ann Neurol. 2021 Jan;89(1):13-23. doi: 10.1002/ana.25927. Epub 2020 Nov 4. Ann Neurol. 2021. PMID: 33091175 Free PMC article. Review.
-
Anti-CD20 Agents for Multiple Sclerosis: Spotlight on Ocrelizumab and Ofatumumab.Brain Sci. 2020 Oct 20;10(10):758. doi: 10.3390/brainsci10100758. Brain Sci. 2020. PMID: 33092190 Free PMC article. Review.
-
Targeting the Brain with Single-Domain Antibodies: Greater Potential Than Stated So Far?Int J Mol Sci. 2023 Jan 30;24(3):2632. doi: 10.3390/ijms24032632. Int J Mol Sci. 2023. PMID: 36768953 Free PMC article. Review.
References
-
- Bar-Or A, Calabresi PA, Arnold D, et al. . Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol 2008;63:395–400. - PubMed
-
- Hauser SL, Waubant E, Arnold DL, et al. . B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358:676–688. - PubMed
-
- Kappos L, Li D, Calabresi PA, et al. . Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet 2011;378:1779–1787. - PubMed
-
- Hauser SL, Bar-Or A, Comi G, et al. . Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017;376:221–234. - PubMed
-
- Bleeker WK, Munk ME, Mackus WJ, et al. . Estimation of dose requirements for sustained in vivo activity of a therapeutic human anti-CD20 antibody. Br J Haematol 2008;140:303–312. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
