Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer
- PMID: 29686424
- PMCID: PMC5964608
- DOI: 10.1038/s41591-018-0007-9
Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer
Erratum in
-
Author Correction: Mechanisms and clinical activity of an EGFR and HER2 exon 20-selective kinase inhibitor in non-small cell lung cancer.Nat Med. 2024 Sep;30(9):2694-2695. doi: 10.1038/s41591-024-03178-1. Nat Med. 2024. PMID: 39164519 No abstract available.
Abstract
Although most activating mutations of epidermal growth factor receptor (EGFR)-mutant non-small cell lung cancers (NSCLCs) are sensitive to available EGFR tyrosine kinase inhibitors (TKIs), a subset with alterations in exon 20 of EGFR and HER2 are intrinsically resistant and lack an effective therapy. We used in silico, in vitro, and in vivo testing to model structural alterations induced by exon 20 mutations and to identify effective inhibitors. 3D modeling indicated alterations restricted the size of the drug-binding pocket, limiting the binding of large, rigid inhibitors. We found that poziotinib, owing to its small size and flexibility, can circumvent these steric changes and is a potent inhibitor of the most common EGFR and HER2 exon 20 mutants. Poziotinib demonstrated greater activity than approved EGFR TKIs in vitro and in patient-derived xenograft models of EGFR or HER2 exon 20 mutant NSCLC and in genetically engineered mouse models of NSCLC. In a phase 2 trial, the first 11 patients with NSCLC with EGFR exon 20 mutations receiving poziotinib had a confirmed objective response rate of 64%. These data identify poziotinib as a potent, clinically active inhibitor of EGFR and HER2 exon 20 mutations and illuminate the molecular features of TKIs that may circumvent steric changes induced by these mutations.
Conflict of interest statement
J.P.R., M.B.N., and J.V.H. have filed patent applications under the Patent Cooperation Treaty and in Taiwan. J.V.H. has had grant or research support from AstraZeneca, Bayer, and GlaxoSmithKline and has served on advisory committees for AstraZeneca, Boehringer Ingelheim, Exelixis, Genentech, GSK, Lilly, Novartis, Spectrum, and Synta. R.C.D. has licensing fees, honorarium, and travel expenses from Ariad Pharmaceuticals, has a Sponsored Research Agreement from Threshold Pharmaceuticals, and has served as an advisory Board member for AstraZeneca.
Figures

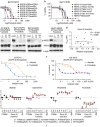
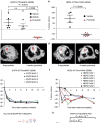
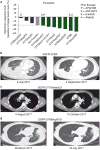
Comment in
-
Poziotinib for uncommon ERBB mutations.Nat Rev Clin Oncol. 2018 Jul;15(7):404. doi: 10.1038/s41571-018-0038-7. Nat Rev Clin Oncol. 2018. PMID: 29743681 No abstract available.
Similar articles
-
EGFR-D770>GY and Other Rare EGFR Exon 20 Insertion Mutations with a G770 Equivalence Are Sensitive to Dacomitinib or Afatinib and Responsive to EGFR Exon 20 Insertion Mutant-Active Inhibitors in Preclinical Models and Clinical Scenarios.Cells. 2021 Dec 17;10(12):3561. doi: 10.3390/cells10123561. Cells. 2021. PMID: 34944068 Free PMC article.
-
Activity of a novel HER2 inhibitor, poziotinib, for HER2 exon 20 mutations in lung cancer and mechanism of acquired resistance: An in vitro study.Lung Cancer. 2018 Dec;126:72-79. doi: 10.1016/j.lungcan.2018.10.019. Epub 2018 Oct 17. Lung Cancer. 2018. PMID: 30527195
-
Structural, biochemical, and clinical characterization of epidermal growth factor receptor (EGFR) exon 20 insertion mutations in lung cancer.Sci Transl Med. 2013 Dec 18;5(216):216ra177. doi: 10.1126/scitranslmed.3007205. Sci Transl Med. 2013. PMID: 24353160 Free PMC article.
-
Novel drugs targeting EGFR and HER2 exon 20 mutations in metastatic NSCLC.Crit Rev Oncol Hematol. 2020 Apr;148:102906. doi: 10.1016/j.critrevonc.2020.102906. Epub 2020 Feb 11. Crit Rev Oncol Hematol. 2020. PMID: 32109716 Review.
-
Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors in non-small cell lung cancer harboring uncommon EGFR mutations: Focus on afatinib.Semin Oncol. 2019 Jun;46(3):271-283. doi: 10.1053/j.seminoncol.2019.08.004. Epub 2019 Sep 11. Semin Oncol. 2019. PMID: 31558282 Review.
Cited by
-
Response to Afatinib in a Patient with NSCLC Harboring Novel EGFR Exon 20 Insertion Mutations.Onco Targets Ther. 2020 Sep 30;13:9753-9757. doi: 10.2147/OTT.S268694. eCollection 2020. Onco Targets Ther. 2020. PMID: 33061454 Free PMC article.
-
Poor effect of osimertinib on EGFR exon 20 insertion-positive lung adenocarcinoma: A case report.Medicine (Baltimore). 2020 Oct 16;99(42):e22628. doi: 10.1097/MD.0000000000022628. Medicine (Baltimore). 2020. PMID: 33080698 Free PMC article.
-
[Chinese Expert Consensus on Next Generation Sequencing Diagnosis for Non-small Cell Lung Cancer (2020 Edition)].Zhongguo Fei Ai Za Zhi. 2020 Sep 20;23(9):741-761. doi: 10.3779/j.issn.1009-3419.2020.101.45. Zhongguo Fei Ai Za Zhi. 2020. PMID: 32957170 Free PMC article. Chinese.
-
A Novel HER2-Selective Kinase Inhibitor Is Effective in HER2 Mutant and Amplified Non-Small Cell Lung Cancer.Cancer Res. 2022 Apr 15;82(8):1633-1645. doi: 10.1158/0008-5472.CAN-21-2693. Cancer Res. 2022. PMID: 35149586 Free PMC article.
-
Advanced non-small-cell lung cancer: how to manage EGFR and HER2 exon 20 insertion mutation-positive disease.Drugs Context. 2022 Jul 29;11:2022-3-9. doi: 10.7573/dic.2022-3-9. eCollection 2022. Drugs Context. 2022. PMID: 35975031 Free PMC article. Review.
References
-
- Bezjak A, et al. Symptom improvement in lung cancer patients treated with erlotinib: quality of life analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2006;24:3831–3837. - PubMed
-
- Rosell R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. - PubMed
-
- Wheatley-Price P, Ding K, Seymour L, Clark GM, Shepherd FA. Erlotinib for advanced non-small-cell lung cancer in the elderly: an analysis of the National Cancer Institute of Canada Clinical Trials Group Study BR.21. J Clin Oncol. 2008;26:2350–2357. - PubMed
-
- Zhou C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

