Monomeric Intermediates Formed by Vesiculovirus Glycoprotein during Its Low-pH-induced Structural Transition
- PMID: 29678555
- PMCID: PMC7126088
- DOI: 10.1016/j.jmb.2018.04.015
Monomeric Intermediates Formed by Vesiculovirus Glycoprotein during Its Low-pH-induced Structural Transition
Abstract
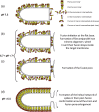
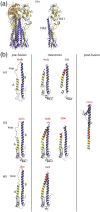
Vesiculoviruses enter cells by membrane fusion, driven by a large, low-pH-induced, conformational change in the fusion glycoprotein (G) that involves transition from a trimeric pre-fusion to a trimeric post-fusion state. G is the model of class III fusion glycoproteins which also includes the fusion glycoproteins of herpesviruses (gB) and baculoviruses (gp64). Class III fusion proteins combine features of the previously characterized class I and class II fusion proteins. In this review, we first present and discuss the data that indicate that the Vesiculovirus G structural transition proceeds through monomeric intermediates. Then, we focus on a recently determined crystal structure of the Chandipura virus G ectodomain that contained two monomeric intermediate conformations of the glycoprotein, revealing the chronological order of the structural changes in the protein and offering a detailed pathway for the conformational change, in agreement with electron microscopy data. In the crystal, the intermediates were associated through their fusion domain in an antiparallel manner to form an intermolecular β-sheet. Mutagenesis indicated that this interface is functionally relevant. All those structural data challenge the current model proposed for viral membrane fusion. Therefore, we wonder if this mode of operating is specific to Vesiculovirus G and discuss data indicating that class II fusion glycoproteins are monomeric when they interact with the target membrane but also crystal structures suggesting the existence of non-trimeric intermediates for influenza hemagglutinin which is the prototype of class I fusion proteins.
Keywords: Influenza; Vesiculovirus; conformational change; glycoprotein; membrane fusion.
Figures
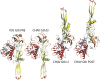

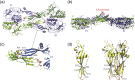
Similar articles
-
Structural intermediates in the fusion-associated transition of vesiculovirus glycoprotein.EMBO J. 2017 Mar 1;36(5):679-692. doi: 10.15252/embj.201694565. Epub 2017 Feb 10. EMBO J. 2017. PMID: 28188244 Free PMC article.
-
Characterization of monomeric intermediates during VSV glycoprotein structural transition.PLoS Pathog. 2012 Feb;8(2):e1002556. doi: 10.1371/journal.ppat.1002556. Epub 2012 Feb 23. PLoS Pathog. 2012. PMID: 22383886 Free PMC article.
-
Crystallization and preliminary X-ray analysis of Chandipura virus glycoprotein G.Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012 Sep 1;68(Pt 9):1094-7. doi: 10.1107/S1744309112030151. Epub 2012 Aug 31. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2012. PMID: 22949203 Free PMC article.
-
Role of electrostatic repulsion in controlling pH-dependent conformational changes of viral fusion proteins.Structure. 2013 Jul 2;21(7):1085-96. doi: 10.1016/j.str.2013.05.009. Structure. 2013. PMID: 23823327 Free PMC article. Review.
-
Intermediate conformations during viral fusion glycoprotein structural transition.Curr Opin Virol. 2013 Apr;3(2):143-50. doi: 10.1016/j.coviro.2013.03.006. Epub 2013 Apr 3. Curr Opin Virol. 2013. PMID: 23562213 Free PMC article. Review.
Cited by
-
Viral Membrane Fusion: A Dance Between Proteins and Lipids.Annu Rev Virol. 2023 Sep 29;10(1):139-161. doi: 10.1146/annurev-virology-111821-093413. Annu Rev Virol. 2023. PMID: 37774128 Free PMC article. Review.
-
Transient opening of trimeric prefusion RSV F proteins.Nat Commun. 2019 May 8;10(1):2105. doi: 10.1038/s41467-019-09807-5. Nat Commun. 2019. PMID: 31068578 Free PMC article.
-
Visualizing intermediate stages of viral membrane fusion by cryo-electron tomography.Trends Biochem Sci. 2024 Oct;49(10):916-931. doi: 10.1016/j.tibs.2024.06.012. Epub 2024 Jul 24. Trends Biochem Sci. 2024. PMID: 39054240 Review.
-
Crystal structure of Mokola virus glycoprotein in its post-fusion conformation.PLoS Pathog. 2020 Mar 9;16(3):e1008383. doi: 10.1371/journal.ppat.1008383. eCollection 2020 Mar. PLoS Pathog. 2020. PMID: 32150590 Free PMC article.
-
The phenuivirus Toscana virus makes an atypical use of vacuolar acidity to enter host cells.PLoS Pathog. 2023 Aug 14;19(8):e1011562. doi: 10.1371/journal.ppat.1011562. eCollection 2023 Aug. PLoS Pathog. 2023. PMID: 37578957 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

