Oral and sublingual immunotherapy for egg allergy
- PMID: 29676439
- PMCID: PMC6494514
- DOI: 10.1002/14651858.CD010638.pub3
Oral and sublingual immunotherapy for egg allergy
Abstract
Background: Clinical egg allergy is a common food allergy. Current management relies upon strict allergen avoidance. Oral immunotherapy might be an optional treatment, through desensitization to egg allergen.
Objectives: To determine the efficacy and safety of oral and sublingual immunotherapy in children and adults with immunoglobulin E (IgE)-mediated egg allergy as compared to a placebo treatment or an avoidance strategy.
Search methods: We searched 13 databases for journal articles, conference proceedings, theses and trials registers using a combination of subject headings and text words (last search 31 March 2017).
Selection criteria: We included randomized controlled trials (RCTs) comparing oral immunotherapy or sublingual immunotherapy administered by any protocol with placebo or an elimination diet. Participants were children or adults with clinical egg allergy.
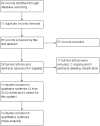
Data collection and analysis: We retrieved 97 studies from the electronic searches. We selected studies, extracted data and assessed the methodological quality. We attempted to contact the study investigators to obtain the unpublished data, wherever possible. We used the I² statistic to assess statistical heterogeneity. We estimated a pooled risk ratio (RR) with 95% confidence interval (CI) for each outcome using a Mantel-Haenzel fixed-effect model if statistical heterogeneity was low (I² value less than 50%). We rated the quality of evidence for all outcomes using GRADE.
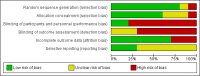
Main results: We included 10 RCTs that met our inclusion criteria, that involved a total of 439 children (oral immunotherapy 249; control intervention 190), aged 1 year to 18 years. Each study used a different oral immunotherapy protocol; none used sublingual immunotherapy. Three studies used placebo and seven used an egg avoidance diet as the control. Primary outcomes were: an increased amount of egg that can be ingested and tolerated without adverse events while receiving allergen-specific oral immunotherapy or sublingual immunotherapy, compared to control; and a complete recovery from egg allergy after completion of oral immunotherapy or sublingual immunotherapy, compared to control. Most children (82%) in the oral immunotherapy group could ingest a partial serving of egg (1 g to 7.5 g) compared to 10% of control group children (RR 7.48, 95% CI 4.91 to 11.38; RD 0.73, 95% CI 0.67 to 0.80). Fewer than half (45%) of children receiving oral immunotherapy were able to tolerate a full serving of egg compared to 10% of the control group (RR 4.25, 95% CI 2.77 to 6.53; RD 0.35, 95% CI 0.28 to 0.43). All 10 trials reported numbers of children with serious adverse events (SAEs) and numbers of children with mild-to-severe adverse events. SAEs requiring epinephrine/adrenaline presented in 21/249 (8.4%) of children in the oral immunotherapy group, and none in the control group. Mild-to-severe adverse events were frequent; 75% of children presented mild-to-severe adverse events during oral immunotherapy treatment versus 6.8% of the control group (RR 8.35, 95% CI 5.31 to 13.12). Of note, seven studies used an egg avoidance diet as the control. Adverse events occurred in 4.2% of children, which may relate to accidental ingestion of egg-containing food. Three studies used a placebo control with adverse events present in 2.6% of children. Overall, there was inconsistent methodological rigour in the trials. All studies enrolled small numbers of children and used different methods to provide oral immunotherapy. Eight included studies were judged to be at high risk of bias in at least one domain. Furthermore, the quality of evidence was judged to be low due to small numbers of participants and events, and possible biases.
Authors' conclusions: Frequent and increasing exposure to egg over one to two years in people who are allergic to egg builds tolerance, with almost everyone becoming more tolerant compared with a minority in the control group and almost half of people being totally tolerant of egg by the end of treatment compared with 1 in 10 people who avoid egg. However, nearly all who received treatment experienced adverse events, mainly allergy-related. We found that 1 in 12 children had serious allergic reactions requiring adrenaline, and some people gave up oral immunotherapy. It appears that oral immunotherapy for egg allergy is effective, but confidence in the trade-off between benefits and harms is low; because there was a small number of trials with few participants, and methodological problems with some trials.
Conflict of interest statement
Olga Romantsik declares she has no competing conflicts of interest.
Maria Grazia Calevo declares she has no competing conflicts of interest.
Maria Angela Tosca declares she has no competing conflicts of interest.
Simona Zappettini declares she has no competing conflicts of interest.
Figures





Update of
-
Oral and sublingual immunotherapy for egg allergy.Cochrane Database Syst Rev. 2014 Nov 18;(11):CD010638. doi: 10.1002/14651858.CD010638.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Apr 20;4:CD010638. doi: 10.1002/14651858.CD010638.pub3. PMID: 25405335 Updated. Review.
Similar articles
-
Oral and sublingual immunotherapy for egg allergy.Cochrane Database Syst Rev. 2014 Nov 18;(11):CD010638. doi: 10.1002/14651858.CD010638.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2018 Apr 20;4:CD010638. doi: 10.1002/14651858.CD010638.pub3. PMID: 25405335 Updated. Review.
-
Allergen-specific oral immunotherapy for peanut allergy.Cochrane Database Syst Rev. 2012 Sep 12;2012(9):CD009014. doi: 10.1002/14651858.CD009014.pub2. Cochrane Database Syst Rev. 2012. PMID: 22972130 Free PMC article. Review.
-
Oral immunotherapy for milk allergy.Cochrane Database Syst Rev. 2012 Nov 14;11(11):CD009542. doi: 10.1002/14651858.CD009542.pub2. Cochrane Database Syst Rev. 2012. PMID: 23152278 Free PMC article. Review.
-
Immunotherapy (oral and sublingual) for food allergy to fruits.Cochrane Database Syst Rev. 2015 Nov 9;2015(11):CD010522. doi: 10.1002/14651858.CD010522.pub2. Cochrane Database Syst Rev. 2015. PMID: 26558953 Free PMC article. Review.
-
Sublingual immunotherapy for asthma.Cochrane Database Syst Rev. 2020 Sep 14;9(9):CD011293. doi: 10.1002/14651858.CD011293.pub3. Cochrane Database Syst Rev. 2020. PMID: 32926419 Free PMC article.
Cited by
-
CSACI guidelines for the ethical, evidence-based and patient-oriented clinical practice of oral immunotherapy in IgE-mediated food allergy.Allergy Asthma Clin Immunol. 2020 Mar 18;16:20. doi: 10.1186/s13223-020-0413-7. eCollection 2020. Allergy Asthma Clin Immunol. 2020. PMID: 32206067 Free PMC article. Review.
-
Immunological Outcomes of Allergen-Specific Immunotherapy in Food Allergy.Front Immunol. 2020 Nov 3;11:568598. doi: 10.3389/fimmu.2020.568598. eCollection 2020. Front Immunol. 2020. PMID: 33224138 Free PMC article. Review.
-
Food allergy: an updated review on pathogenesis, diagnosis, prevention and management.Acta Biomed. 2020 Sep 15;91(11-S):e2020012. doi: 10.23750/abm.v91i11-S.10316. Acta Biomed. 2020. PMID: 33004782 Free PMC article. Review.
-
Guided Gradual Egg-Tolerance Induction in Hen's Egg Allergic Children Tolerating Baked Egg: A Prospective Randomized Trial.Front Allergy. 2022 May 11;3:886094. doi: 10.3389/falgy.2022.886094. eCollection 2022. Front Allergy. 2022. PMID: 35769568 Free PMC article.
-
Tolerance to heated egg in egg allergy: Explanations and implications for prevention and treatment.Clin Transl Allergy. 2023 Dec;13(12):e12312. doi: 10.1002/clt2.12312. Clin Transl Allergy. 2023. PMID: 38146801 Free PMC article. Review.
References
References to studies included in this review
Akashi 2017 {published data only}
-
- Akashi M, Yasudo H, Narita M, Nomura I, Akasawa A, Ebisawa M, et al. Randomized controlled trial of oral immunotherapy for egg allergy in Japanese patients. Pediatrics International 2017;59(5):534‐9. - PubMed
Burks 2012 {published data only}
Caminiti 2015 {published data only}
-
- Caminiti L, Pajno GB, Crisafulli G, Chiera F, Collura M, Panasci G, et al. Oral immunotherapy for egg allergy: a double‐blind placebo‐controlled study, with postdesensitization follow‐up. Journal of Allergy and Clinical Immunology: In Practice 2015;3(4):532‐9. - PubMed
Dello Iacono 2013 {published data only}
-
- Dello Iacono I, Tripodi S, Calvani M, Panetta V, Verga MC, Miceli Sopo S. Specific oral tolerance induction with raw hen’s egg in children with very severe egg allergy: A randomized controlled trial. Pediatric Allergy and Immunology 2013;24(1):66‐74. - PubMed
Escudero 2015 {published data only}
-
- Escudero C, Rodrıguez del Rıo P, Sánchez‐Garcia S, Pérez‐Rangel I, Pérez‐Farinós N, Garcia‐Fernández C, et al. Early sustained unresponsiveness after short‐course egg oral immunotherapy: a randomized controlled study in egg‐allergic children. Clinical and Experimental Allergy 2015;45(12):1833‐43. - PubMed
Fuentes‐Aparicio 2013 {published data only}
-
- Fuentes‐Aparicio V, Alvarez‐Perea A, Infante S, Zapatero L, D’Oleo A, Alonso‐Lebrero E. Specific oral tolerance induction in paediatric patients with persistent egg allergy. Allergologia et Immunopathologia 2013;41(3):143‐50. - PubMed
Giavi 2016 {published data only}
-
- Giavi S, Vissers YM, Muraro A, Lauener R, Konstantinopoulos AP, Mercenier A. Oral immunotherapy with low allergenic hydrolysed egg in egg allergic children. European Journal of Allergy and Clinical Immunology 2016;71(11):1575‐84. - PubMed
Meglio 2013 {published data only}
-
- Meglio P, Giampietro PG, Carello R, Gabriele I, Avitabile S, Galli E. Oral food desensitization in children with IgE‐mediated hen’s egg allergy:a new protocol with raw hen’s egg. Pediatric Allergy and Immunology 2013;24(1):75‐83. - PubMed
Pérez‐Rangel 2017 {published data only}
-
- Pérez‐Rangel I, Rodríguez Del Río P, Escudero C, Sánchez‐García S, Sánchez‐Hernández JJ, Ibáñez MD. Efficacy and safety of high‐dose rush oral immunotherapy in persistent egg allergic children: A randomized clinical trial. Annals of Allergy, Asthma & Immunology 2017;118(3):356‐64. - PubMed
Vazquez‐Ortiz 2014 {published data only}
-
- Vazquez‐Ortiz M, Alvaro M, Piquer M, Dominguez O, Machinena A, Martın‐Mateos MA, et al. Baseline specific IgE levels are useful to predict safety of oral immunotherapy in egg‐allergic children. Clinical and Experimental Allergy 2014;44(1):130‐41. - PubMed
References to studies excluded from this review
Buchanan 2007 {published data only}
-
- Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in non anaphylactic children with egg allergy. Journal of Allergy and Clinical Immunology 2007;119(1):199‐205. - PubMed
García Rodríguez 2011 {published data only}
-
- García Rodríguez R, Urra JM, Feo‐Brito F, Galindo PA, Borja J, Gómez E, et al. Oral rush desensitization to egg: efficacy and safety. Clinical and Experimental Allergy 2011;41(9):1289‐96. - PubMed
Itoh 2010 {published data only}
-
- Itoh N, Itagaki Y, Kurihara K. Rush specific oral tolerance induction in school‐age children with severe egg allergy: One year follow up. Allergology International 2010;59(1):43‐51. - PubMed
Meglio 2011 {published data only}
-
- Meglio P, Giampietro PG, Gabriele I, Carello R, Avitabile S, Galli E. Oral desensitisation with food is food‐specific and protein‐specific. International Journal of Immunopathology and Pharmacology 2011;24(3):803‐11. - PubMed
Morisset 2007 {published data only}
-
- Morisset M, Moneret‐Vautrin DA, Guenard L, Cuny JM, Frentz P, Hatahet R, et al. Oral desensitization in children with milk and egg allergies obtains recovery in a significant proportion of cases. A randomized study in 60 children with cow's milk allergy and 90 children with egg allergy. European Annals of Allergy and Clinical Immunology 2007;39(1):12‐9. - PubMed
Palmer 2013 {published data only}
-
- Palmer DJ, Metcalfe J, Makrides M, Gold MS, Quinn P, West CE, et al. Early regular egg exposure in infants with eczema: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2013;132(2):387‐92.e1. - PubMed
Patriarca 1998 {published data only}
-
- Patriarca G, Schiavino D, Nucera E, Schinco G, Milani A, Gasbarrini GB. Food allergy in children: results of a standardized protocol for oral desensitization. Hepatogastroenterology 1998;45(19):52‐8. - PubMed
Patriarca 2007 {published data only}
-
- Patriarca G, Nucera E, Pollastrini E, Roncallo C, Pasquale T, Lombardo C, et al. Oral specific desensitization in food‐allergic children. Digestive Diseases and Sciences 2007;52(7):1662‐72. - PubMed
Ruiz Garcia 2012 {published data only}
-
- Ruiz Garcia M, Haroun E, Landivar ME, Torres Hernandez JA, Sastre J. Commercial dehydrated egg white for specific oral tolerance induction (SOTI): an easier treatment for egg allergy. Journal of Investigational Allergology and Clinical Immunology 2012;22(7):529‐31. - PubMed
Staden 2007 {published data only}
-
- Staden U, Rolinck‐Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: efficacy and clinical patterns of reaction. Allergy 2007;62(11):1261‐9. - PubMed
References to studies awaiting assessment
Hirajama 2016 {published data only}
-
- Hirayama J, Nagao M, Fujisawa T, Itoh‐Nagato N, Shimojo N, Iwata T. The long‐term prognosis of oral immunotherapy for eggs and cow's milk allergy. European Journal of Allergy and Clinical Immunology. Conference: 35th Annual Congress of the European Academy of Allergy and Clinical Immunology, EAACI 2016. Blackwell Publishing Ltd, August 2016; Vol. 71:501.
Itoh‐Nagato 2013 {published data only}
-
- Itoh‐Nagato N, Fujisawa T, Shimojo N, Nagao M, Iwata T. High rate of desensitisation and tolerance induction by rush oral immunotherapy for anaphylactic children with egg allergy: a randomised controlled trial. Allergy 2013;68(Suppl 97):337‐497.
Pajno 2012 {published data only}
-
- Pajno GB, Caminiti L, Vita D, Crisafulli G. Oral immunotherapy for egg allergy. Clinical and immunologic results. Journal of Allergy and Clinical Immunology 2012;129(Suppl 2):AB51. - PubMed
Takahashi 2013 {published data only}
-
- Takahashi M, Taniuchi S, Soejima K, Hatano Y, Nakano K, Kaneko K. The effectiveness and safety of specific oral immunotherapy in children with food allergy at stratification. Allergy 2013;68(Suppl 97):189‐336.
Yanagida 2013 {published data only}
-
- Yanagida N, Minoura T, Ebisawa M. Oral immunotherapy at fixed low dose for mild to moderate hen's egg allergy. Journal of Allergy and Clinical Immunology 2013;31(Suppl 2):AB84.
References to ongoing studies
NCT01846208 {published data only}
-
- Oral desensitization to egg with subsequent induction of sustained unresponsiveness for egg‐allergic children using baked egg or egg oral immunotherapy. Ongoing study July 2013.
NCT02083471 {published data only}
-
- Cow's milk and hen's egg hyposensitization in adults. Ongoing study April 2015.
Additional references
Burks 2008
-
- Burks AW. Peanut allergy. Lancet 2008;371(9623):1538‐46. - PubMed
Burks 2012a
-
- Burks AW, Tang M, Sicherer S, Muraro A, Eigenmann PA, Ebisawa M, et al. ICON: food allergy. Journal of Allergy and Clinical Immunology 2012;129(4):906‐20. - PubMed
Christie 2002
-
- Christie L, Hine RJ, Parker JG, Burks W. Food allergies in children affect nutrient intake and growth. Journal of the American Dietetic Association 2002;102(11):1648‐51. - PubMed
Cummings 2010
-
- Cummings AJ, Knibb RC, King RM, Lucas JS. The psychosocial impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy 2010;65(8):933‐45. - PubMed
Fleischer 2012
Galli 2010
GRADEpro GDT [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Ibanez Sandin MD 2017 [pers comm]
-
- Ibanez Sandin MD. Safety data regarding first five month of ROIT1 [personal communication]. Email to: O Romantsik 12 February 2017.
Jones 2009
Jones 2016
Khoriaty 2013
Land 2011
Leonard 2012
Muraro 2014
-
- Muraro A, Werfel T, Hoffman‐Sommergruber K, Roberts G, Beyer K, Bindslev‐Jensen C, et al. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy Asthma Proceedings 2014;69(8):1008‐25. - PubMed
Nelson 1997
-
- Nelson HS, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract. Journal of Allergy and Clinical Immunology 1997;99(6 Pt 1):744‐51. - PubMed
Nurmatov 2017
Oppenheimer 1992
-
- Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DY. Treatment of peanut allergy with rush immunotherapy. Journal of Allergy and Clinical Immunology 1992;90(2):256‐62. - PubMed
Osborne 2011
-
- Osborne NJ, Koplin JJ, Martin PE, Gurrin LC, Lowe AJ, Matheson MC, et al. HealthNuts Investigators. Prevalence of challenge‐proven IgE‐mediated food allergy using population‐based sampling and predetermined challenge criteria in infants. Journal of Allergy and Clinical Immunology 2011;127(3):668‐76. - PubMed
Patriarca 1984
-
- Patriarca C, Romano A, Venuti A, Schiavino D, Rienzo V, Nucera E, et al. Oral specific hyposensitization in the management of patients allergic to food. Allergologia et Immunopathologia 1984;12(4):275‐81. - PubMed
Patriarca 2003
-
- Patriarca G, Nucera E, Roncallo C, Pollastrini E, Bartolozzi F, Pasquale T, et al. Oral desensitizing treatment in food allergy: clinical and immunological results. Alimentary Pharmacology and Therapeutics 2003;17(3):459‐65. [Erratum in: Alimentary Pharmacology and Therapeutics. 2003 May 1;17(9):1205] - PubMed
Ring 2011
-
- Rin J, Gutermuth J. 100 years of hyposensitization: history of allergen‐specific immunotherapy (ASIT). Allergy 2011;66(6):713‐24. - PubMed
Sampson 2003
-
- Sampson HA. Anaphylaxis and emergency treatment. Pediatrics 2003;111:1601‐8. - PubMed
Sampson 2012
-
- Sampson HA, Gerth van Wijk R, Bindslev‐Jensen C, Sicherer S, Teuber SS, Burks AW, et al. Standardizing double‐blind, placebo‐controlled oral food challenges: American Academy of Allergy, Asthma & Immunology‐European Academy of Allergy and Clinical Immunology PRACTALL consensus report. Journal of Allergy and Clinical Immunology 2012;130(6):1260‐74. - PubMed
Savage 2007
-
- Savage JH, Matsui EC, Skripak JM, Wood RA. The natural history of egg allergy. Journal of Allergy and Clinical Immunology 2007;120(6):1413‐7. - PubMed
Sclar 2014
-
- Sclar DA, Lieberman PL. Anaphylaxis: underdiagnosed, underreported, and undertreated. American Journal of Medicine 2014;127(1 Suppl):S1‐5. - PubMed
Sicherer 2010
Simons 2009
-
- Simons FE. Anaphylaxis: Recent advances in assessment and treatment. Allergy and Clinical Immunology 2009;124(4):625‐36. - PubMed
Simons 2011
-
- Simons FE. Anaphylaxis pathogenesis and treatment. Allergy 2011;66(Suppl 95):31‐4. - PubMed
Wood 2003b
-
- Wood RA. The natural history of food allergy. Pediatrics 2003;111(6 Pt 3):1631‐7. - PubMed
References to other published versions of this review
Romantsik 2013
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

