Histone demethylase JMJD1A coordinates acute and chronic adaptation to cold stress via thermogenic phospho-switch
- PMID: 29674659
- PMCID: PMC5908789
- DOI: 10.1038/s41467-018-03868-8
Histone demethylase JMJD1A coordinates acute and chronic adaptation to cold stress via thermogenic phospho-switch
Abstract
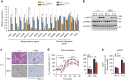
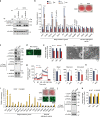
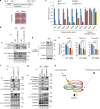
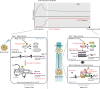
In acute cold stress in mammals, JMJD1A, a histone H3 lysine 9 (H3K9) demethylase, upregulates thermogenic gene expressions through β-adrenergic signaling in brown adipose tissue (BAT). Aside BAT-driven thermogenesis, mammals have another mechanism to cope with long-term cold stress by inducing the browning of the subcutaneous white adipose tissue (scWAT). Here, we show that this occurs through a two-step process that requires both β-adrenergic-dependent phosphorylation of S265 and demethylation of H3K9me2 by JMJD1A. The histone demethylation-independent acute Ucp1 induction in BAT and demethylation-dependent chronic Ucp1 expression in beige scWAT provides complementary molecular mechanisms to ensure an ordered transition between acute and chronic adaptation to cold stress. JMJD1A mediates two major signaling pathways, namely, β-adrenergic receptor and peroxisome proliferator-activated receptor-γ (PPARγ) activation, via PRDM16-PPARγ-P-JMJD1A complex for beige adipogenesis. S265 phosphorylation of JMJD1A, and the following demethylation of H3K9me2 might prove to be a novel molecular target for the treatment of metabolic disorders, via promoting beige adipogenesis.
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
[β-Adrenergic Signaling Regulates a Concerted Thermogenic Response in Brown Adipose Tissue and White Adipose Tissue].Brain Nerve. 2022 Feb;74(2):151-158. doi: 10.11477/mf.1416202000. Brain Nerve. 2022. PMID: 35108679 Japanese.
-
Cold acclimation and pioglitazone combined increase thermogenic capacity of brown and white adipose tissues but this does not translate into higher energy expenditure in mice.Am J Physiol Endocrinol Metab. 2023 Apr 1;324(4):E358-E373. doi: 10.1152/ajpendo.00217.2022. Epub 2023 Mar 1. Am J Physiol Endocrinol Metab. 2023. PMID: 36856189
-
JMJD1A is a signal-sensing scaffold that regulates acute chromatin dynamics via SWI/SNF association for thermogenesis.Nat Commun. 2015 May 7;6:7052. doi: 10.1038/ncomms8052. Nat Commun. 2015. PMID: 25948511 Free PMC article.
-
Histone demethylases regulate adipocyte thermogenesis.Diabetol Int. 2018 Aug 16;9(4):215-223. doi: 10.1007/s13340-018-0366-y. eCollection 2018 Oct. Diabetol Int. 2018. PMID: 30603371 Free PMC article. Review.
-
β-Adrenergic Signal and Epigenomic Regulatory Process for Adaptive Thermogenesis.Adv Exp Med Biol. 2024;1461:213-227. doi: 10.1007/978-981-97-4584-5_15. Adv Exp Med Biol. 2024. PMID: 39289284 Review.
Cited by
-
Investigating transcriptome-wide sex dimorphism by multi-level analysis of single-cell RNA sequencing data in ten mouse cell types.Biol Sex Differ. 2020 Nov 5;11(1):61. doi: 10.1186/s13293-020-00335-2. Biol Sex Differ. 2020. PMID: 33153500 Free PMC article.
-
Epitranscriptomics in metabolic disease.Nat Metab. 2023 Mar;5(3):370-384. doi: 10.1038/s42255-023-00764-4. Epub 2023 Mar 23. Nat Metab. 2023. PMID: 36959512 Review.
-
Brown Adipose Tissue-A Translational Perspective.Endocr Rev. 2023 Mar 4;44(2):143-192. doi: 10.1210/endrev/bnac015. Endocr Rev. 2023. PMID: 35640259 Free PMC article. Review.
-
KDM3A regulates alternative splicing of cell-cycle genes following DNA damage.RNA. 2021 Nov;27(11):1353-1362. doi: 10.1261/rna.078796.121. Epub 2021 Jul 28. RNA. 2021. PMID: 34321328 Free PMC article.
-
The Different Shades of Thermogenic Adipose Tissue.Curr Obes Rep. 2024 Sep;13(3):440-460. doi: 10.1007/s13679-024-00559-y. Epub 2024 Apr 12. Curr Obes Rep. 2024. PMID: 38607478 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

