Structure based drug design and in vitro metabolism study: Discovery of N-(4-methylthiophenyl)-N,2-dimethyl-cyclopenta[d]pyrimidine as a potent microtubule targeting agent
- PMID: 29655610
- PMCID: PMC5967629
- DOI: 10.1016/j.bmc.2018.04.010
Structure based drug design and in vitro metabolism study: Discovery of N-(4-methylthiophenyl)-N,2-dimethyl-cyclopenta[d]pyrimidine as a potent microtubule targeting agent
Abstract
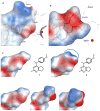
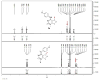
We report a series of tubulin targeting agents, some of which demonstrate potent antiproliferative activities. These analogs were designed to optimize the antiproliferative activity of 1 by varying the heteroatom substituent at the 4'-position, the basicity of the 4-position amino moiety, and conformational restriction. The potential metabolites of the active compounds were also synthesized. Some compounds demonstrated single digit nanomolar IC50 values for antiproliferative effects in MDA-MB-435 melanoma cells. Particularly, the S-methyl analog 3 was more potent than 1 in MDA-MB-435 cells (IC50 = 4.6 nM). Incubation of 3 with human liver microsomes showed that the primary metabolite of the S-methyl moiety of 3 was the methyl sulfinyl group, as in analog 5. This metabolite was equipotent with the lead compound 1 in MDA-MB-435 cells (IC50 = 7.9 nM). Molecular modeling and electrostatic surface area were determined to explain the activities of the analogs. Most of the potent compounds overcome multiple mechanisms of drug resistance and compound 3 emerged as the lead compound for further SAR and preclinical development.
Keywords: Cyclopenta[d]pyrimidine; Metabolism; Microtubule targeting agent.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Notes
The authors declare no competing financial interest.
Figures




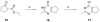
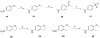

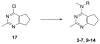
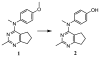
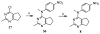
Similar articles
-
Design, Synthesis, and Biological Evaluation of 5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidines as Microtubule Targeting Agents.Molecules. 2022 Jan 5;27(1):321. doi: 10.3390/molecules27010321. Molecules. 2022. PMID: 35011550 Free PMC article.
-
Design, Synthesis and Molecular Docking Studies of Novel Indole-Pyrimidine Hybrids as Tubulin Polymerization Inhibitors.Chem Biol Drug Des. 2015 Dec;86(6):1491-500. doi: 10.1111/cbdd.12616. Epub 2015 Aug 3. Chem Biol Drug Des. 2015. PMID: 26177395
-
Synthesis and biological evaluation of novel indole-pyrimidine hybrids bearing morpholine and thiomorpholine moieties.Eur J Med Chem. 2017 Jul 7;134:110-118. doi: 10.1016/j.ejmech.2017.04.011. Epub 2017 Apr 7. Eur J Med Chem. 2017. PMID: 28410492
-
Design, synthesis, and bioevaluation of pyrazolo[1,5-a]pyrimidine derivatives as tubulin polymerization inhibitors targeting the colchicine binding site with potent anticancer activities.Eur J Med Chem. 2020 Sep 15;202:112519. doi: 10.1016/j.ejmech.2020.112519. Epub 2020 Jun 27. Eur J Med Chem. 2020. PMID: 32650183
-
Tubulin-targeting agents in hybrid drugs.Curr Med Chem. 2010;17(7):609-39. doi: 10.2174/092986710790416254. Curr Med Chem. 2010. PMID: 20088764 Review.
Cited by
-
The 3-D conformational shape of N-naphthyl-cyclopenta[d]pyrimidines affects their potency as microtubule targeting agents and their antitumor activity.Bioorg Med Chem. 2021 Jan 1;29:115887. doi: 10.1016/j.bmc.2020.115887. Epub 2020 Nov 24. Bioorg Med Chem. 2021. PMID: 33310545 Free PMC article.
-
Design, Synthesis, and Biological Evaluation of 5,6,7,8-Tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidines as Microtubule Targeting Agents.Molecules. 2022 Jan 5;27(1):321. doi: 10.3390/molecules27010321. Molecules. 2022. PMID: 35011550 Free PMC article.
-
Synthesis and Pharmacological Evaluation of Noncatechol G Protein Biased and Unbiased Dopamine D1 Receptor Agonists.ACS Med Chem Lett. 2019 Apr 5;10(5):792-799. doi: 10.1021/acsmedchemlett.9b00050. eCollection 2019 May 9. ACS Med Chem Lett. 2019. Retraction in: ACS Med Chem Lett. 2022 May 17;13(6):989. doi: 10.1021/acsmedchemlett.2c00197. PMID: 31098001 Free PMC article. Retracted.
-
Discovery of amide-bridged pyrrolo[2,3-d]pyrimidines as tumor targeted classical antifolates with selective uptake by folate receptor α and inhibition of de novo purine nucleotide biosynthesis.Bioorg Med Chem. 2019 Dec 1;27(23):115125. doi: 10.1016/j.bmc.2019.115125. Epub 2019 Oct 17. Bioorg Med Chem. 2019. PMID: 31679978 Free PMC article.
References
-
- Saez-Calvo G, Sharma A, Balaguer FA, et al. Triazolopyrimidines are microtubule-stabilizing agents that bind the vinca inhibitor site of tubulin. Cell chemical biology. 2017;24(6):737–750. e736. - PubMed
-
- Madiraju C, Edler MC, Hamel E, et al. Tubulin assembly, taxoid site binding, and cellular effects of the microtubule-stabilizing agent dictyostatin. Biochemistry. 2005;44(45):15053–15063. - PubMed
-
- Jang SH, Wientjes MG, Au JLS. Determinants of paclitaxel uptake, accumulation and retention in solid tumors. Invest New Drugs. 2001;19(2):113–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

