Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury
- PMID: 29650962
- PMCID: PMC5938045
- DOI: 10.1038/s12276-018-0055-8
Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury
Abstract
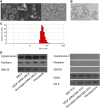
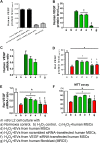
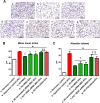
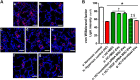
We previously reported the role of vascular endothelial growth factor (VEGF) secreted by mesenchymal stem cells (MSCs) in protecting against neonatal hyperoxic lung injuries. Recently, the paracrine protective effect of MSCs was reported to be primarily mediated by extracellular vesicle (EV) secretion. However, the therapeutic efficacy of MSC-derived EVs and the role of the VEGF contained within EVs in neonatal hyperoxic lung injury have not been elucidated. The aim of the study was to determine whether MSC-derived EVs attenuate neonatal hyperoxic lung injury and, if so, whether this protection is mediated via the transfer of VEGF. We compared the therapeutic efficacy of MSCs, MSC-derived EVs with or without VEGF knockdown, and fibroblast-derived EVs in vitro with a rat lung epithelial cell line challenged with H2O2 and in vivo with newborn Sprague-Dawley rats exposed to hyperoxia (90%) for 14 days. MSCs (1 × 105 cells) or EVs (20 µg) were administered intratracheally on postnatal day 5. The MSCs and MSC-derived EVs, but not the EVs derived from VEGF-knockdown MSCs or fibroblasts, attenuated the in vitro H2O2-induced L2 cell death and the in vivo hyperoxic lung injuries, such as impaired alveolarization and angiogenesis, increased cell death, and activated macrophages and proinflammatory cytokines. PKH67-stained EVs were internalized into vascular pericytes (22.7%), macrophages (21.3%), type 2 epithelial cells (19.5%), and fibroblasts (4.4%) but not into vascular endothelial cells. MSC-derived EVs are as effective as parental MSCs for attenuating neonatal hyperoxic lung injuries, and this protection was mediated primarily by the transfer of VEGF.
Conflict of interest statement
Samsung Medical Center and MEDIPOST Co., Ltd. own issued or filed patents for “Method of treating lung diseases using cells separated or proliferated from umbilical cord blood” in the names of the inventors Y.S.C. and W.S.P. The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Critical role of vascular endothelial growth factor secreted by mesenchymal stem cells in hyperoxic lung injury.Am J Respir Cell Mol Biol. 2014 Sep;51(3):391-9. doi: 10.1165/rcmb.2013-0385OC. Am J Respir Cell Mol Biol. 2014. PMID: 24669883
-
Cell type-dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury.Cytotherapy. 2015 Aug;17(8):1025-35. doi: 10.1016/j.jcyt.2015.03.008. Epub 2015 Apr 8. Cytotherapy. 2015. PMID: 25863963
-
Mesenchymal stem cell-derived extracellular vesicles attenuate pulmonary vascular permeability and lung injury induced by hemorrhagic shock and trauma.J Trauma Acute Care Surg. 2018 Feb;84(2):245-256. doi: 10.1097/TA.0000000000001744. J Trauma Acute Care Surg. 2018. PMID: 29251710 Free PMC article.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury.Int J Mol Sci. 2021 Jun 20;22(12):6596. doi: 10.3390/ijms22126596. Int J Mol Sci. 2021. PMID: 34202940 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Novel Cell-Free Therapy for Sepsis.Front Immunol. 2020 Apr 21;11:647. doi: 10.3389/fimmu.2020.00647. eCollection 2020. Front Immunol. 2020. PMID: 32373121 Free PMC article. Review.
Cited by
-
Mesenchymal Stromal Cells Primed by Toll-like Receptors 3 and 4 Enhanced Anti-Inflammatory Effects against LPS-Induced Macrophages via Extracellular Vesicles.Int J Mol Sci. 2023 Nov 13;24(22):16264. doi: 10.3390/ijms242216264. Int J Mol Sci. 2023. PMID: 38003458 Free PMC article.
-
Mesenchymal Stromal/Stem Cell Extracellular Vesicles and Perinatal Injury: One Formula for Many Diseases.Stem Cells. 2022 Nov 29;40(11):991-1007. doi: 10.1093/stmcls/sxac062. Stem Cells. 2022. PMID: 36044737 Free PMC article. Review.
-
VEGF Contributes to Mesenchymal Stem Cell-Mediated Reversion of Nor1-Dependent Hypertrophy in iPS Cell-Derived Cardiomyocytes.Stem Cells Int. 2021 Apr 10;2021:8888575. doi: 10.1155/2021/8888575. eCollection 2021. Stem Cells Int. 2021. PMID: 33927770 Free PMC article.
-
Adipose tissue-derived small extracellular vesicles modulate macrophages to improve the homing of adipocyte precursors and endothelial cells in adipose tissue regeneration.Front Cell Dev Biol. 2022 Dec 6;10:1075233. doi: 10.3389/fcell.2022.1075233. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36561367 Free PMC article.
-
Extracellular vesicles: Potential impact on cardiovascular diseases.Adv Clin Chem. 2021;105:49-100. doi: 10.1016/bs.acc.2021.02.002. Epub 2021 Mar 10. Adv Clin Chem. 2021. PMID: 34809830 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

