Long-Term Functional and Structural Consequences of Primary Blast Overpressure to the Eye
- PMID: 29648979
- PMCID: PMC6098409
- DOI: 10.1089/neu.2017.5394
Long-Term Functional and Structural Consequences of Primary Blast Overpressure to the Eye
Abstract
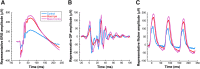
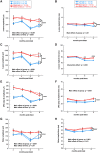
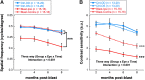
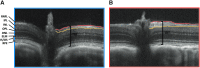
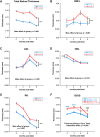
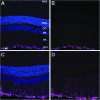
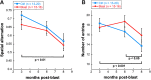
Acoustic blast overpressure (ABO) injury in military personnel and civilians is often accompanied by delayed visual deficits. However, most animal model studies dealing with blast-induced visual defects have focused on short-term (≤1 month) changes. Here, we evaluated long-term (≤8 months) retinal structure and function deficits in rats with ABO injury. Adult male Long-Evans rats were subjected to ABO from a single blast (approximately 190 dB SPL, ∼63 kPa, @80 psi), generated by a shock tube device. Retinal function (electroretinography; ERG), visual function (optomotor response), retinal thickness (spectral domain-optical coherence tomography; SD-OCT), and spatial cognition/exploratory motor behavior (Y-maze) were measured at 2, 4, 6, and 8 months post-blast. Immunohistochemical analysis of glial fibrillary acidic protein (GFAP) in retinal sections was performed at 8 months post-blast. Electroretinogram a- and b-waves, oscillatory potentials, and flicker responses showed greater amplitudes with delayed implicit times in both eyes of blast-exposed animals, relative to controls. Contrast sensitivity (CS) was reduced in both eyes of blast-exposed animals, whereas spatial frequency (SF) was decreased only in ipsilateral eyes, relative to controls. Total retinal thickness was greater in both eyes of blast-exposed animals, relative to controls, due to increased thickness of several retinal layers. Age, but not blast exposure, altered Y-maze outcomes. GFAP was greatly increased in blast-exposed retinas. ABO exposure resulted in visual and retinal changes that persisted up to 8 months post-blast, mimicking some of the visual deficits observed in human blast-exposed patients, thereby making this a useful model to study mechanisms of injury and potential treatments.
Keywords: blast; electroretinogram; optomotor response; retina; visual function.
Conflict of interest statement
No competing financial interests exist.
Figures

Similar articles
-
Blast Exposure Induces Ocular Functional Changes with Increasing Blast Over-pressures in a Rat Model.Curr Eye Res. 2019 Jul;44(7):770-780. doi: 10.1080/02713683.2019.1567791. Curr Eye Res. 2019. PMID: 30947563
-
Dependence of visual and cognitive outcomes on animal holder configuration in a rodent model of blast overpressure exposure.Vision Res. 2021 Nov;188:162-173. doi: 10.1016/j.visres.2021.07.008. Epub 2021 Jul 30. Vision Res. 2021. PMID: 34333201 Free PMC article.
-
Retinal gliosis and phenotypic diversity of intermediate filament induction and remodeling upon acoustic blast overpressure (ABO) exposure to the rat eye.Exp Eye Res. 2023 Sep;234:109585. doi: 10.1016/j.exer.2023.109585. Epub 2023 Jul 21. Exp Eye Res. 2023. PMID: 37481225 Free PMC article.
-
Blast injury: Impact to the cornea.Exp Eye Res. 2024 Jul;244:109915. doi: 10.1016/j.exer.2024.109915. Epub 2024 Apr 25. Exp Eye Res. 2024. PMID: 38677709 Review.
-
The pathology of primary blast overpressure injury.Toxicology. 1997 Jul 25;121(1):17-28. doi: 10.1016/s0300-483x(97)03652-4. Toxicology. 1997. PMID: 9217312 Review.
Cited by
-
Blast-Mediated Traumatic Brain Injury Exacerbates Retinal Damage and Amyloidosis in the APPswePSENd19e Mouse Model of Alzheimer's Disease.Invest Ophthalmol Vis Sci. 2019 Jun 3;60(7):2716-2725. doi: 10.1167/iovs.18-26353. Invest Ophthalmol Vis Sci. 2019. PMID: 31247112 Free PMC article.
-
Visual Outcomes in Experimental Rodent Models of Blast-Mediated Traumatic Brain Injury.Front Mol Neurosci. 2021 Apr 15;14:659576. doi: 10.3389/fnmol.2021.659576. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33935648 Free PMC article. Review.
-
Model matters: Differential outcomes in traumatic optic neuropathy pathophysiology between blunt and blast-wave mediated head injuries.Exp Neurol. 2024 Feb;372:114613. doi: 10.1016/j.expneurol.2023.114613. Epub 2023 Nov 22. Exp Neurol. 2024. PMID: 37995952
-
Identification of chronic brain protein changes and protein targets of serum auto-antibodies after blast-mediated traumatic brain injury.Heliyon. 2020 Feb 17;6(2):e03374. doi: 10.1016/j.heliyon.2020.e03374. eCollection 2020 Feb. Heliyon. 2020. PMID: 32099918 Free PMC article.
-
Behavioral Assessment of Visual Function via Optomotor Response and Cognitive Function via Y-Maze in Diabetic Rats.J Vis Exp. 2020 Oct 23;(164):10.3791/61806. doi: 10.3791/61806. J Vis Exp. 2020. PMID: 33165321 Free PMC article.
References
-
- Okie S. (2005). Traumatic brain injury in the war zone. N. Engl. J. Med. 352, 2043–2047 - PubMed
-
- Defence and Veterans Brain Injury Center. DoD worldwide numbers for TBI. Available at: http://www.dvbic.org/dod-worldwide-numbers-tbi Accessed July14, 2017
-
- Goodrich G.L., Martinsen G.L., Flyg H.M., Kirby J., Garvert D.W., and Tyler C.W. (2014). Visual function, traumatic brain injury, and posttraumatic stress disorder. J. Rehabil. Res. Dev. 51, 547–558 - PubMed
-
- Cockerham G.C., Goodrich G.L., Weichel E.D., Orcutt J.C., Rizzo J.F., Bower K.S., and Schuchard R.A. (2009). Eye and visual function in traumatic brain injury. J. Rehabil. Res. Dev. 46, 811–818 - PubMed
-
- Cockerham G.C., Rice T.A., Hewes E.H., Cockerham K.P., Lemke S., Wang G., Lin R.C., Glynn-Milley C. and Zumhagen L. (2011). Closed-eye ocular injuries in the Iraq and Afghanistan wars. N. Engl. J. Med. 364, 2172–2173 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

