Age is not just a number: Naive T cells increase their ability to persist in the circulation over time
- PMID: 29641514
- PMCID: PMC5894957
- DOI: 10.1371/journal.pbio.2003949
Age is not just a number: Naive T cells increase their ability to persist in the circulation over time
Abstract
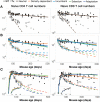
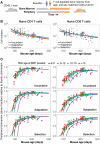
The processes regulating peripheral naive T-cell numbers and clonal diversity remain poorly understood. Conceptually, homeostatic mechanisms must fall into the broad categories of neutral (simple random birth-death models), competition (regulation of cell numbers through quorum-sensing, perhaps via limiting shared resources), adaptation (involving cell-intrinsic changes in homeostatic fitness, defined as net growth rate over time), or selection (involving the loss or outgrowth of cell populations deriving from intercellular variation in fitness). There may also be stably maintained heterogeneity within the naive T-cell pool. To distinguish between these mechanisms, we confront very general models of these processes with an array of experimental data, both new and published. While reduced competition for homeostatic stimuli may impact cell survival or proliferation in neonates or under moderate to severe lymphopenia, we show that the only mechanism capable of explaining multiple, independent experimental studies of naive CD4+ and CD8+ T-cell homeostasis in mice from young adulthood into old age is one of adaptation, in which cells act independently and accrue a survival or proliferative advantage continuously with their post-thymic age. However, aged naive T cells may also be functionally impaired, and so the accumulation of older cells via 'conditioning through experience' may contribute to reduced immune responsiveness in the elderly.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Cell generation dynamics underlying naive T-cell homeostasis in adult humans.PLoS Biol. 2019 Oct 29;17(10):e3000383. doi: 10.1371/journal.pbio.3000383. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31661488 Free PMC article.
-
A major role for memory CD4 T cells in the control of lymphopenia-induced proliferation of naive CD4 T cells.J Immunol. 2005 May 1;174(9):5316-23. doi: 10.4049/jimmunol.174.9.5316. J Immunol. 2005. PMID: 15843528
-
Differential lymphopenia-induced homeostatic proliferation for CD4+ and CD8+ T cells following septic injury.J Leukoc Biol. 2009 Mar;85(3):382-90. doi: 10.1189/jlb.0808491. Epub 2008 Dec 30. J Leukoc Biol. 2009. PMID: 19088177 Free PMC article.
-
Cytokine signals in T-cell homeostasis.J Immunother. 2005 Jul-Aug;28(4):289-94. doi: 10.1097/01.cji.0000165356.03924.e7. J Immunother. 2005. PMID: 16000945 Review.
-
Fratricide: a mechanism for T memory-cell homeostasis.Trends Immunol. 2003 Jul;24(7):370-5. doi: 10.1016/s1471-4906(03)00164-9. Trends Immunol. 2003. PMID: 12860527 Review.
Cited by
-
The dynamics and longevity of circulating CD4+ memory T cells depend on cell age and not the chronological age of the host.PLoS Biol. 2024 Aug 13;22(8):e3002380. doi: 10.1371/journal.pbio.3002380. eCollection 2024 Aug. PLoS Biol. 2024. PMID: 39137219 Free PMC article.
-
The dynamics and longevity of circulating CD4+ memory T cells depend on cell age and not the chronological age of the host.bioRxiv [Preprint]. 2024 Jun 25:2023.10.16.562650. doi: 10.1101/2023.10.16.562650. bioRxiv. 2024. Update in: PLoS Biol. 2024 Aug 13;22(8):e3002380. doi: 10.1371/journal.pbio.3002380. PMID: 38948729 Free PMC article. Updated. Preprint.
-
Quantifying cellular dynamics in mice using a novel fluorescent division reporter system.Front Immunol. 2023 Jul 27;14:1157705. doi: 10.3389/fimmu.2023.1157705. eCollection 2023. Front Immunol. 2023. PMID: 37575229 Free PMC article.
-
Modeling T Cell Fate.Annu Rev Immunol. 2023 Apr 26;41:513-532. doi: 10.1146/annurev-immunol-101721-040924. Annu Rev Immunol. 2023. PMID: 37126420 Free PMC article. Review.
-
Effect of cellular aging on memory T-cell homeostasis.Front Immunol. 2022 Aug 8;13:947242. doi: 10.3389/fimmu.2022.947242. eCollection 2022. Front Immunol. 2022. PMID: 36059495 Free PMC article.
References
-
- Palmer DB. The Effect of Age on Thymic Function. Front Immunol. 2013;4:316 doi: 10.3389/fimmu.2013.00316 - DOI - PMC - PubMed
-
- Hale JS, Boursalian TE, Turk GL, Fink PJ. Thymic Output in Aged Mice. Proc Natl Acad Sci USA. 2006;103(22):8447–8452. doi: 10.1073/pnas.0601040103 - DOI - PMC - PubMed
-
- Tanchot C, Lemonnier FA, Pérarnau B, Freitas AA, Rocha B. Differential Requirements for Survival and Proliferation of CD8 Nave or Memory T Cells. Science. 1997;276(5321):2057–2062. doi: 10.1126/science.276.5321.2057 - DOI - PubMed
-
- Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC Class II Molecules Are Not Required for Survival of Newly Generated CD4+ T Cells, but Affect Their Long-Term Life Span. Immunity. 1996;5(3):217–228. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

