Risk-Benefit Assessment of Ethinylestradiol Using a Physiologically Based Pharmacokinetic Modeling Approach
- PMID: 29637542
- PMCID: PMC6282492
- DOI: 10.1002/cpt.1085
Risk-Benefit Assessment of Ethinylestradiol Using a Physiologically Based Pharmacokinetic Modeling Approach
Abstract
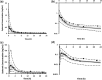
Current formulations of combined oral contraceptives (COC) containing ethinylestradiol (EE) have ≤35 μg due to increased risks of cardiovascular diseases (CVD) with higher doses of EE. Low-dose formulations however, have resulted in increased incidences of breakthrough bleeding and contraceptive failure, particularly when coadministered with inducers of cytochrome P450 enzymes (CYP). The developed physiologically based pharmacokinetic model quantitatively predicted the effect of CYP3A4 inhibition and induction on the pharmacokinetics of EE. The predicted Cmax and AUC ratios when coadministered with voriconazole, fluconazole, rifampicin, and carbamazepine were within 1.25 of the observed data. Based on published clinical data, an AUCss value of 1,000 pg/ml.h was selected as the threshold for breakthrough bleeding. Prospective application of the model in simulations of different doses of EE (20 μg, 35 μg, and 50 μg) identified percentages of the population at risk of breakthrough bleeding alone and with varying degrees of CYP modulation.
© 2018 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals, Inc. on behalf of American Society for Clinical Pharmacology and Therapeutics.
Figures

Similar articles
-
The Effects of Weak and Strong CYP3A Induction by Rifampicin on the Pharmacokinetics of Five Progestins and Ethinylestradiol Compared to Midazolam.Clin Pharmacol Ther. 2020 Oct;108(4):798-807. doi: 10.1002/cpt.1848. Epub 2020 May 11. Clin Pharmacol Ther. 2020. PMID: 32275771 Free PMC article. Clinical Trial.
-
Quantitative Assessment of Levonorgestrel Binding Partner Interplay and Drug-Drug Interactions Using Physiologically Based Pharmacokinetic Modeling.CPT Pharmacometrics Syst Pharmacol. 2021 Jan;10(1):48-58. doi: 10.1002/psp4.12572. Epub 2020 Dec 13. CPT Pharmacometrics Syst Pharmacol. 2021. PMID: 33217171 Free PMC article.
-
Pharmacokinetic drug interactions involving 17alpha-ethinylestradiol: a new look at an old drug.Clin Pharmacokinet. 2007;46(2):133-57. doi: 10.2165/00003088-200746020-00003. Clin Pharmacokinet. 2007. PMID: 17253885 Review.
-
A comparison of the pharmacokinetic profile of an ascending-dose, extended-regimen combined oral contraceptive to those of other extended regimens.Reprod Sci. 2014 Nov;21(11):1401-10. doi: 10.1177/1933719114526472. Epub 2014 Mar 19. Reprod Sci. 2014. PMID: 24647707
-
Ethinyl estradiol and 17β-estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment.Contraception. 2013 Jun;87(6):706-27. doi: 10.1016/j.contraception.2012.12.011. Epub 2013 Jan 8. Contraception. 2013. PMID: 23375353 Review.
Cited by
-
Physiologically-based pharmacokinetic modeling of prominent oral contraceptive agents and applications in drug-drug interactions.CPT Pharmacometrics Syst Pharmacol. 2024 Apr;13(4):563-575. doi: 10.1002/psp4.13101. Epub 2024 Jan 15. CPT Pharmacometrics Syst Pharmacol. 2024. PMID: 38130003 Free PMC article.
-
Combined Oral Contraceptives As Victims of Drug Interactions.Drug Metab Dispos. 2023 Jun;51(6):718-732. doi: 10.1124/dmd.122.000854. Epub 2023 Mar 24. Drug Metab Dispos. 2023. PMID: 36963837 Free PMC article. Review.
References
-
- Smyth, C. Contraception Failure Blamed for Abortions. <https://www.thetimes.co.uk/article/contraception-failure-blamed-for-abor...> (2017). Accessed 7 July 2017.
-
- Chasan‐Taber, L. & Stampfer, M.J. Epidemiology of oral contraceptives and cardiovascular disease. Ann. Intern. Med. 128, 467–477 (1998). - PubMed
-
- U.S. Food & Drug Administration . Labeling for Combined Hormonal Contraceptives: Guidance for Industry. <https://www.fda.gov/ucm/groups/fdagov-public/@fdagov-drugs-gen/documents...> (2017). Accessed 1 December 2017.
-
- Stanczyk, F.Z. , Archer, D.F. & Bhavnani, B.R. Ethinyl estradiol and 17beta‐estradiol in combined oral contraceptives: pharmacokinetics, pharmacodynamics and risk assessment. Contraception 87, 706–727 (2013). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

