Pharmacological regulation of outflow resistance distal to Schlemm's canal
- PMID: 29631366
- PMCID: PMC6087729
- DOI: 10.1152/ajpcell.00024.2018
Pharmacological regulation of outflow resistance distal to Schlemm's canal
Abstract
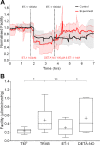
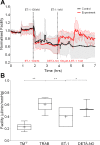
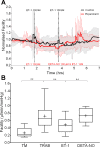
The trabecular meshwork (TM) and Schlemm's canal generate the majority of outflow resistance; however, the distal regions of the conventional outflow pathway account for 25-50% of total resistance. Sections of distal vessels are surrounded by α-smooth muscle actin-containing cells, indicating that they may be vasoregulated. This study examined the effect of a potent vasodilator, nitric oxide (NO), and its physiological antagonist, endothelin-1 (ET-1), on the regulation of outflow resistance in the distal regions of the conventional outflow pathway. Using a physiological model of the conventional outflow pathway, human and porcine anterior segments were perfused in organ culture under constant flow conditions, while intrachamber pressure was continually monitored. For porcine anterior segments, a stable baseline outflow facility with TM intact was first achieved before anterior segments were removed and a trabeculotomy was performed. For human anterior segments, a trabeculotomy was immediately performed. In human anterior segments, 100 nM ET-1 significantly decreased distal outflow facility from 0.49 ± 0.26 to 0.31 ± 0.18 (mean ± SD) µl·min-1·mmHg, P < 0.01. Perfusion with 100 µM diethylenetriamine-NO in the presence of 1 nM ET-1 immediately reversed ET-1 effects, significantly increasing distal outflow facility to 0.54 ± 0.35 µl·min-1·mmHg, P = 0.01. Similar results were obtained in porcine anterior segment experiments. Therefore, data show a dynamic range of resistance generation by distal vessels in both the human and the porcine conventional outflow pathways. Interestingly, maximal contraction of vessels in the distal outflow tract of trabeculotomized eyes generated resistance very near physiological levels for both species having an intact TM.
Keywords: aqueous humor; distal outflow; glaucoma; outflow physiology.
Figures

Similar articles
-
NO-induced regulation of human trabecular meshwork cell volume and aqueous humor outflow facility involve the BKCa ion channel.Am J Physiol Cell Physiol. 2008 Jun;294(6):C1378-86. doi: 10.1152/ajpcell.00363.2007. Epub 2008 Apr 2. Am J Physiol Cell Physiol. 2008. PMID: 18385281
-
Morphological changes to Schlemm's canal and the distal aqueous outflow pathway in monkey eyes with laser-induced ocular hypertension.Exp Eye Res. 2022 Jun;219:109030. doi: 10.1016/j.exer.2022.109030. Epub 2022 Mar 10. Exp Eye Res. 2022. PMID: 35283108 Free PMC article.
-
Pharmacologic disruption of Schlemm's canal cells and outflow facility in anterior segments of human eyes.Invest Ophthalmol Vis Sci. 2004 Jul;45(7):2246-54. doi: 10.1167/iovs.03-0746. Invest Ophthalmol Vis Sci. 2004. PMID: 15223802
-
Pressure-induced expression changes in segmental flow regions of the human trabecular meshwork.Exp Eye Res. 2017 May;158:67-72. doi: 10.1016/j.exer.2016.06.009. Epub 2016 Jun 19. Exp Eye Res. 2017. PMID: 27334250 Free PMC article. Review.
-
Evaluation of neural innervation in the human conventional outflow pathway distal to Schlemm's canal.Exp Eye Res. 2022 Aug;221:109132. doi: 10.1016/j.exer.2022.109132. Epub 2022 May 27. Exp Eye Res. 2022. PMID: 35636488 Free PMC article. Review.
Cited by
-
Modeling the biomechanics of the conventional aqueous outflow pathway microstructure in the human eye.Comput Methods Programs Biomed. 2022 Jun;221:106922. doi: 10.1016/j.cmpb.2022.106922. Epub 2022 May 29. Comput Methods Programs Biomed. 2022. PMID: 35660940 Free PMC article.
-
Aqueous outflow regulation - 21st century concepts.Prog Retin Eye Res. 2021 Jul;83:100917. doi: 10.1016/j.preteyeres.2020.100917. Epub 2020 Nov 17. Prog Retin Eye Res. 2021. PMID: 33217556 Free PMC article. Review.
-
Structure-Function Changes of the Porcine Distal Outflow Tract in Response to Nitric Oxide.Invest Ophthalmol Vis Sci. 2018 Oct 1;59(12):4886-4895. doi: 10.1167/iovs.18-24943. Invest Ophthalmol Vis Sci. 2018. PMID: 30347083 Free PMC article.
-
VIP Induces Changes in the F-/G-Actin Ratio of Schlemm's Canal Endothelium via LRRK2 Transcriptional Regulation.Invest Ophthalmol Vis Sci. 2020 Jun 3;61(6):45. doi: 10.1167/iovs.61.6.45. Invest Ophthalmol Vis Sci. 2020. PMID: 32572455 Free PMC article.
-
Physiologic Consequences of Caveolin-1 Ablation in Conventional Outflow Endothelia.Invest Ophthalmol Vis Sci. 2020 Sep 1;61(11):32. doi: 10.1167/iovs.61.11.32. Invest Ophthalmol Vis Sci. 2020. PMID: 32940661 Free PMC article.
References
-
- Borghi V, Bastia E, Guzzetta M, Chiroli V, Toris CB, Batugo MR, Carreiro ST, Chong WK, Gale DC, Kucera DJ, Jia L, Prasanna G, Ongini E, Krauss AH, Impagnatiello F. A novel nitric oxide releasing prostaglandin analog, NCX 125, reduces intraocular pressure in rabbit, dog, and primate models of glaucoma. J Ocul Pharmacol Ther 26: 125–132, 2010. doi:10.1089/jop.2009.0120. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

