Use of Anti-phospho-girdin Antibodies to Visualize Intestinal Tuft Cells in Free-Floating Mouse Jejunum Cryosections
- PMID: 29630055
- PMCID: PMC5933235
- DOI: 10.3791/57475
Use of Anti-phospho-girdin Antibodies to Visualize Intestinal Tuft Cells in Free-Floating Mouse Jejunum Cryosections
Abstract
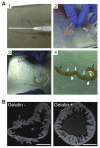
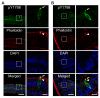
The actin binding protein girdin is a cytosolic protein that is required for actin remodeling to trigger cell migration in various tissues. Girdin is phosphorylated by both receptor and non-receptor tyrosine kinases at tyrosine 1798. Omori et al. developed site- and phosphorylation status-specific antibodies against human girdin at tyrosine-1798 (pY1798), which specifically bind to phosphorylated tyrosine-1798, but not to unphosphorylated tyrosine-1798. pY1798 antibodies have been used to specifically label tuft cells (TCs) that are present in mammalian gastrointestinal tissues, but the function of these cells is unclear. This protocol allows the robust visualization of TCs in the jejunum using pY1798 antibodies and immunofluorescence. To ensure successful and simple TC visualization, this protocol includes two histological techniques: production of free-floating cryosections from gelatin-filled jejunum tissue, and low-temperature antigen retrieval at 50 °C for 3 h. Filling the jejunum with gelatin maintains the shape of free-floating sections throughout the staining procedure, whereas low-temperature antigen retrieval ensures robust signals from TCs. Successful use of this protocol results in pY1798 staining of TCs distributed from villus tip to crypt. Stained TCs have a spool-shaped soma and fluorescent signals condense at the lumenal tip, which corresponds to the protruding 'tuft.' Phalloidin staining colocalized with pY1798-positive TCs at the thickened brush border, and corresponds to a rootlet mass extending from the TC tuft. This protocol could be used to examine TCs in human biopsy samples collected with gastrointestinal endoscopes. Furthermore, TCs were recently reported to accumulate following parasite infection in mice, suggesting that this protocol could have applications for diagnosis of parasite infections in the human gut.
Similar articles
-
Tyrosine Phosphorylation of an Actin-Binding Protein Girdin Specifically Marks Tuft Cells in Human and Mouse Gut.J Histochem Cytochem. 2017 Jun;65(6):347-366. doi: 10.1369/0022155417702586. Epub 2017 Apr 4. J Histochem Cytochem. 2017. PMID: 28375676 Free PMC article.
-
Girdin is phosphorylated on tyrosine 1798 when associated with structures required for migration.Biochem Biophys Res Commun. 2015 Mar 20;458(4):934-40. doi: 10.1016/j.bbrc.2015.02.065. Epub 2015 Feb 20. Biochem Biophys Res Commun. 2015. PMID: 25707853
-
Distribution of duodenal tuft cells is altered in pediatric patients with acute and chronic enteropathy.Biomed Res. 2020;41(2):113-118. doi: 10.2220/biomedres.41.113. Biomed Res. 2020. PMID: 32307399 Free PMC article.
-
Girdin, a novel actin-binding protein, and its family of proteins possess versatile functions in the Akt and Wnt signaling pathways.Ann N Y Acad Sci. 2006 Nov;1086:169-84. doi: 10.1196/annals.1377.016. Ann N Y Acad Sci. 2006. PMID: 17185515 Review.
-
[Roles of DISC1-interacting protein Girdin in postnatal development and adult neurogenesis in the dentate gyrus].Nihon Shinkei Seishin Yakurigaku Zasshi. 2011 Feb;31(1):23-8. Nihon Shinkei Seishin Yakurigaku Zasshi. 2011. PMID: 21409841 Review. Japanese.
Cited by
-
Cell differentiation is disrupted by MYO5B loss through Wnt/Notch imbalance.JCI Insight. 2021 Aug 23;6(16):e150416. doi: 10.1172/jci.insight.150416. JCI Insight. 2021. PMID: 34197342 Free PMC article.
-
Single-Cell Imaging of Metastatic Potential of Cancer Cells.iScience. 2018 Dec 21;10:53-65. doi: 10.1016/j.isci.2018.11.022. Epub 2018 Nov 15. iScience. 2018. PMID: 30500482 Free PMC article.
References
-
- Jarvi O, Keyrilainen O. On the cellular structures of the epithelial invasions in the glandular stomach of mice caused by intramural application of 20-methylcholantren. Acta Pathol Microbiol Scand Suppl. 1956;39(Suppl 111):72–73. - PubMed
-
- Enomoto A, et al. Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev Cell. 2005;9(3):389–402. - PubMed
-
- Asai M, et al. Similar phenotypes of Girdin germ-line and conditional knockout mice indicate a crucial role for Girdin in the nestin lineage. Biochem Biophys Res Commun. 2012;426(4):533–538. - PubMed
-
- Kitamura T, et al. Regulation of VEGF-mediated angiogenesis by the Akt/PKB substrate Girdin. Nat Cell Biol. 2008;10(3):329–337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources