U1 snRNP Alteration and Neuronal Cell Cycle Reentry in Alzheimer Disease
- PMID: 29628886
- PMCID: PMC5876301
- DOI: 10.3389/fnagi.2018.00075
U1 snRNP Alteration and Neuronal Cell Cycle Reentry in Alzheimer Disease
Abstract
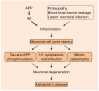
The aberrancy of U1 small nuclear ribonucleoprotein (snRNP) complex and RNA splicing has been demonstrated in Alzheimer's disease (AD). Importantly, the U1 proteopathy is AD-specific, widespread and early-occurring, thus providing a very unique clue to the AD pathogenesis. The prominent feature of U1 histopathology is its nuclear depletion and redistribution in the neuronal cytoplasm. According to the preliminary data, the initial U1 cytoplasmic distribution pattern is similar to the subcellular translocation of the spliceosome in cells undergoing mitosis. This implies that the U1 mislocalization might reflect the neuronal cell cycle-reentry (CCR) which has been extensively evidenced in AD brains. The CCR phenomenon explains the major molecular and cellular events in AD brains, such as Tau and amyloid precursor protein (APP) phosphorylation, and the possible neuronal death through mitotic catastrophe (MC). Furthermore, the CCR might be mechanistically linked to inflammation, a critical factor in the AD etiology according to the genetic evidence. Therefore, the discovery of U1 aberrancy might strengthen the involvement of CCR in the AD neuronal degeneration.
Keywords: Alzheimer’s disease; U1 snRNP; cell cycle reentry; cytoplasmic redistribution; inflammation.
Figures
Similar articles
-
U1 small nuclear ribonucleoprotein complex and RNA splicing alterations in Alzheimer's disease.Proc Natl Acad Sci U S A. 2013 Oct 8;110(41):16562-7. doi: 10.1073/pnas.1310249110. Epub 2013 Sep 10. Proc Natl Acad Sci U S A. 2013. PMID: 24023061 Free PMC article.
-
U1 small nuclear ribonucleoproteins (snRNPs) aggregate in Alzheimer's disease due to autosomal dominant genetic mutations and trisomy 21.Mol Neurodegener. 2014 Apr 28;9:15. doi: 10.1186/1750-1326-9-15. Mol Neurodegener. 2014. PMID: 24773620 Free PMC article.
-
Alzheimer's disease-associated U1 snRNP splicing dysfunction causes neuronal hyperexcitability and cognitive impairment.Nat Aging. 2022 Oct;2(10):923-940. doi: 10.1038/s43587-022-00290-0. Epub 2022 Oct 12. Nat Aging. 2022. PMID: 36636325 Free PMC article.
-
The fate of the U1 snRNP autoantigen during apoptosis: implications for systemic autoimmunity.Isr Med Assoc J. 2002 Sep;4(9):706-12. Isr Med Assoc J. 2002. PMID: 12440236 Review.
-
Deep Profiling of the Aggregated Proteome in Alzheimer's Disease: From Pathology to Disease Mechanisms.Proteomes. 2018 Nov 12;6(4):46. doi: 10.3390/proteomes6040046. Proteomes. 2018. PMID: 30424485 Free PMC article. Review.
Cited by
-
Alternative splicing in Alzheimer's disease.Aging Clin Exp Res. 2021 Apr;33(4):747-758. doi: 10.1007/s40520-019-01360-x. Epub 2019 Oct 3. Aging Clin Exp Res. 2021. PMID: 31583531 Review.
-
U1 snRNP proteins promote proximal alternative polyadenylation sites by directly interacting with 3' end processing core factors.J Mol Cell Biol. 2022 Dec 26;14(8):mjac054. doi: 10.1093/jmcb/mjac054. J Mol Cell Biol. 2022. PMID: 36073763 Free PMC article.
-
U1 snRNA interactions with deep intronic sequences regulate splicing of multiple exons of spinal muscular atrophy genes.Front Neurosci. 2024 Jul 12;18:1412893. doi: 10.3389/fnins.2024.1412893. eCollection 2024. Front Neurosci. 2024. PMID: 39086841 Free PMC article.
-
The Critical Role of Nurr1 as a Mediator and Therapeutic Target in Alzheimer's Disease-related Pathogenesis.Aging Dis. 2020 May 9;11(3):705-724. doi: 10.14336/AD.2019.0718. eCollection 2020 May. Aging Dis. 2020. PMID: 32489714 Free PMC article. Review.
-
Genome-Wide Mapping Implicates 5-Hydroxymethylcytosines in Diabetes Mellitus and Alzheimer's Disease.J Alzheimers Dis. 2023;93(3):1135-1151. doi: 10.3233/JAD-221113. J Alzheimers Dis. 2023. PMID: 37182870 Free PMC article.
References
-
- Arai T., Hasegawa M., Akiyama H., Ikeda K., Nonaka T., Mori H., et al. . (2006). TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem. Biophys. Res. Commun. 351, 602–611. 10.1016/j.bbrc.2006.10.093 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources