Structure Versus Stochasticity-The Role of Molecular Crowding and Intrinsic Disorder in Membrane Fission
- PMID: 29627460
- PMCID: PMC6045432
- DOI: 10.1016/j.jmb.2018.03.024
Structure Versus Stochasticity-The Role of Molecular Crowding and Intrinsic Disorder in Membrane Fission
Abstract
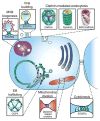
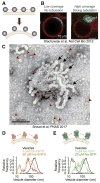
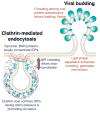
Cellular membranes must undergo remodeling to facilitate critical functions including membrane trafficking, organelle biogenesis, and cell division. An essential step in membrane remodeling is membrane fission, in which an initially continuous membrane surface is divided into multiple, separate compartments. The established view has been that membrane fission requires proteins with conserved structural features such as helical scaffolds, hydrophobic insertions, and polymerized assemblies. In this review, we discuss these structure-based fission mechanisms and highlight recent findings from several groups that support an alternative, structure-independent mechanism of membrane fission. This mechanism relies on lateral collisions among crowded, membrane-bound proteins to generate sufficient steric pressure to drive membrane vesiculation. As a stochastic process, this mechanism contrasts with the paradigm that deterministic protein structures are required to drive fission, raising the prospect that many more proteins may participate in fission than previously thought. Paradoxically, our recent work suggests that intrinsically disordered domains may be among the most potent drivers of membrane fission, owing to their large hydrodynamic radii and substantial chain entropy. This stochastic view of fission also suggests new roles for the structure-based fission proteins. Specifically, we hypothesize that in addition to driving fission directly, the canonical fission machines may facilitate the enrichment and organization of bulky disordered protein domains in order to promote membrane fission by locally amplifying protein crowding.
Keywords: intrinsically disordered proteins; membrane biophysics; membrane fission; membrane traffic; protein crowding.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Membrane fission by protein crowding.Proc Natl Acad Sci U S A. 2017 Apr 18;114(16):E3258-E3267. doi: 10.1073/pnas.1616199114. Epub 2017 Apr 3. Proc Natl Acad Sci U S A. 2017. PMID: 28373566 Free PMC article.
-
BAR scaffolds drive membrane fission by crowding disordered domains.J Cell Biol. 2019 Feb 4;218(2):664-682. doi: 10.1083/jcb.201807119. Epub 2018 Nov 30. J Cell Biol. 2019. PMID: 30504247 Free PMC article.
-
A Tethered Vesicle Assay for High-Throughput Quantification of Membrane Fission.Methods Enzymol. 2018;611:559-582. doi: 10.1016/bs.mie.2018.08.014. Epub 2018 Sep 25. Methods Enzymol. 2018. PMID: 30471700 Free PMC article.
-
Crowd-Sourcing of Membrane Fission: How crowding of non-specialized membrane-bound proteins contributes to cellular membrane fission.Bioessays. 2017 Dec;39(12). doi: 10.1002/bies.201700117. Epub 2017 Oct 20. Bioessays. 2017. PMID: 29052840 Review.
-
Shaping membranes with disordered proteins.Arch Biochem Biophys. 2019 Nov 30;677:108163. doi: 10.1016/j.abb.2019.108163. Epub 2019 Oct 29. Arch Biochem Biophys. 2019. PMID: 31672499 Review.
Cited by
-
The ins and outs of membrane bending by intrinsically disordered proteins.Sci Adv. 2023 Jul 7;9(27):eadg3485. doi: 10.1126/sciadv.adg3485. Epub 2023 Jul 7. Sci Adv. 2023. PMID: 37418523 Free PMC article.
-
Insights into Membrane Curvature Sensing and Membrane Remodeling by Intrinsically Disordered Proteins and Protein Regions.J Membr Biol. 2022 Jun;255(2-3):237-259. doi: 10.1007/s00232-022-00237-x. Epub 2022 Apr 22. J Membr Biol. 2022. PMID: 35451616 Free PMC article. Review.
-
A Förster Resonance Energy Transfer-Based Sensor of Steric Pressure on Membrane Surfaces.J Am Chem Soc. 2020 Dec 9;142(49):20796-20805. doi: 10.1021/jacs.0c09802. Epub 2020 Nov 25. J Am Chem Soc. 2020. PMID: 33237768 Free PMC article.
-
Protein Amphipathic Helix Insertion: A Mechanism to Induce Membrane Fission.Front Cell Dev Biol. 2019 Dec 10;7:291. doi: 10.3389/fcell.2019.00291. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31921835 Free PMC article. Review.
-
Strength in numbers: effect of protein crowding on the shape of cell membranes.Biochem Soc Trans. 2022 Oct 31;50(5):1257-1267. doi: 10.1042/BST20210883. Biochem Soc Trans. 2022. PMID: 36214373 Free PMC article. Review.
References
-
- Conner S, Schmid S. Regulated portals of entry into the cell. Nature. 2003;422:37–44. - PubMed
-
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annual Review of Biochemistry. 2007;76:751–80. - PubMed
-
- Carlton J, Martin-Serrano J. Parallels between cytokinesis and retroviral budding: A role for the ESCRT machinery. Science. 2007;316:1908–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

