Immunotherapy in inflammatory bowel disease: Novel and emerging treatments
- PMID: 29624476
- PMCID: PMC6314405
- DOI: 10.1080/21645515.2018.1461297
Immunotherapy in inflammatory bowel disease: Novel and emerging treatments
Abstract
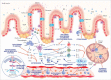
Inflammatory bowel disease (IBD) is a chronic disabling inflammatory process that affects young individuals, with growing incidence. The etiopathogenesis of IBD remains poorly understood. A combination of genetic and environmental factors triggers an inadequate immune response against the commensal intestinal flora in IBD patients. Thus, a better understanding of the immunological mechanisms involved in IBD pathogenesis is central to the development of new therapeutic options. Current pharmacological treatments used in clinical practice like thiopurines or anti-TNF are effective but can produce significant side effects and their efficacy may diminish over time. In fact, up to one third of the patients do not have a satisfactory response to these therapies. Consequently, the search for new therapeutic strategies targeting alternative immunological pathways has intensified. Several new oral and parenteral substances are in the pipeline for IBD. In this review we discuss novel therapies targeting alternative pro-inflammatory pathways like IL-12/23 axis, IL-6 pathway or Janus Kinase inhibitors; as well as others modulating anti-inflammatory signalling pathways like transforming growth factor-β1 (TGF-β1). We also highlight new emerging therapies targeting the adhesion and migration of leukocytes into the inflamed intestinal mucosa by blocking selectively different subunits of α4β7 integrins or binding alternative adhesion molecules like MAdCAM-1. Drugs reducing the circulating lymphocytes by sequestering them in secondary lymphoid organs (sphingosine-1-phosphate (S1P) receptor modulators) are also discussed. Finally, the latest advances in cell therapies using mesenchymal stem cells or engineered T regs are reviewed. In addition, we provide an update on the current status in clinical trials of these new immune-regulating therapies that open a new era in the treatment of IBD.
Keywords: Crohn's disease; Janus kinases; adhesion molecules; cell therapy; inflammatory bowel disease; therapy; ulcerative colitis.
Figures
Similar articles
-
Current, experimental, and future treatments in inflammatory bowel disease: a clinical review.Immunopharmacol Immunotoxicol. 2018 Dec;40(6):446-460. doi: 10.1080/08923973.2018.1469144. Epub 2018 May 10. Immunopharmacol Immunotoxicol. 2018. PMID: 29745777 Review.
-
Biologic therapy for inflammatory bowel disease.Drugs. 2005;65(16):2253-86. doi: 10.2165/00003495-200565160-00002. Drugs. 2005. PMID: 16266194 Review.
-
Novel therapeutic targets for inflammatory bowel disease.J Autoimmun. 2017 Dec;85:103-116. doi: 10.1016/j.jaut.2017.07.004. Epub 2017 Jul 12. J Autoimmun. 2017. PMID: 28711286 Review.
-
Anti-integrin therapy for inflammatory bowel disease.World J Gastroenterol. 2018 May 7;24(17):1868-1880. doi: 10.3748/wjg.v24.i17.1868. World J Gastroenterol. 2018. PMID: 29740202 Free PMC article. Review.
-
Inflammatory pathways of importance for management of inflammatory bowel disease.World J Gastroenterol. 2014 Jan 7;20(1):64-77. doi: 10.3748/wjg.v20.i1.64. World J Gastroenterol. 2014. PMID: 24415859 Free PMC article. Review.
Cited by
-
Improving the Efficacy of Mesenchymal Stem/Stromal-Based Therapy for Treatment of Inflammatory Bowel Diseases.Biomedicines. 2021 Oct 20;9(11):1507. doi: 10.3390/biomedicines9111507. Biomedicines. 2021. PMID: 34829736 Free PMC article. Review.
-
Extracts and Marine Algae Polysaccharides in Therapy and Prevention of Inflammatory Diseases of the Intestine.Mar Drugs. 2020 May 31;18(6):289. doi: 10.3390/md18060289. Mar Drugs. 2020. PMID: 32486405 Free PMC article. Review.
-
Soluble Protein Hydrolysate Ameliorates Gastrointestinal Inflammation and Injury in 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Colitis in Mice.Biomolecules. 2022 Sep 13;12(9):1287. doi: 10.3390/biom12091287. Biomolecules. 2022. PMID: 36139127 Free PMC article.
-
Prevalence of, and factors associated with, long-term COVID-19 sick leave in working-age patients followed in general practices in Germany.Int J Infect Dis. 2021 Aug;109:203-208. doi: 10.1016/j.ijid.2021.06.063. Epub 2021 Jul 2. Int J Infect Dis. 2021. PMID: 34224870 Free PMC article.
-
Conditioned medium of mesenchymal stem cells pretreated with H2O2 promotes intestinal mucosal repair in acute experimental colitis.Sci Rep. 2022 Dec 1;12(1):20772. doi: 10.1038/s41598-022-24493-y. Sci Rep. 2022. PMID: 36456585 Free PMC article.
References
-
- Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, et al.. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology. 2012;142:46–54.e42. doi:10.1053/j.gastro.2011.10.001. PMID:22001864. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
