Hematopoietic cells as site of first-pass catabolism after subcutaneous dosing and contributors to systemic clearance of a monoclonal antibody in mice
- PMID: 29621428
- PMCID: PMC6150636
- DOI: 10.1080/19420862.2018.1458808
Hematopoietic cells as site of first-pass catabolism after subcutaneous dosing and contributors to systemic clearance of a monoclonal antibody in mice
Abstract
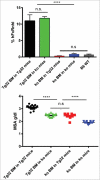
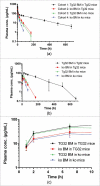
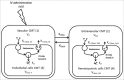
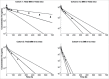
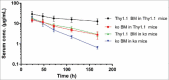
The neonatal Fc receptor (FcRn) has been demonstrated to contribute to a high bioavailability of monoclonal antibodies (mAbs). In this study, we explored the cellular sites of FcRn-mediated protection after subcutaneous (SC) and intravenous (IV) administration. SC absorption and IV disposition kinetics of a mAb were studied in hFcRn transgenic (Tg) bone marrow chimeric mice in which hFcRn was restricted to radioresistant cells or hematopoietic cells. SC bioavailabilities close to 90% were observed in hFcRn Tg mice and chimeric mice with hFcRn expression in hematopoietic cells, whereas SC bioavailabilities were markedly lower when FcRn was missing in hematopoietic cells. Our study demonstrates: 1) FcRn in radiosensitive hematopoietic cells is required for high SC bioavailability, indicating first-pass catabolism after SC administration by hematopoietic cells; 2) FcRn-mediated transcytosis or recycling by radioresistent cells is not required for high SC bioavailability; and 3) after IV administration hematopoietic and radioresistent cells contribute about equally to clearance of the mAb. A pharmacokinetic model was devised to describe a mixed elimination via radioresistent and hematopoietic cells from vascular and extravascular compartments, respectively. Overall, the study indicates a relevant role of hematopoietic cells for first-pass clearance of mAbs after SC administration and confirms their role in the overall clearance of mAbs.
Keywords: FcRn; clearance; hematopoietic cells; subcutaneous first pass catabolism.
Figures
Similar articles
-
Model-Based Assessment of the Contribution of Monocytes and Macrophages to the Pharmacokinetics of Monoclonal Antibodies.Pharm Res. 2022 Feb;39(2):239-250. doi: 10.1007/s11095-022-03177-2. Epub 2022 Feb 3. Pharm Res. 2022. PMID: 35118567
-
Subcutaneous bioavailability of therapeutic antibodies as a function of FcRn binding affinity in mice.MAbs. 2012 Jan-Feb;4(1):101-9. doi: 10.4161/mabs.4.1.18543. MAbs. 2012. PMID: 22327433 Free PMC article.
-
Application of human FcRn transgenic mice as a pharmacokinetic screening tool of monoclonal antibody.Xenobiotica. 2014 Dec;44(12):1127-34. doi: 10.3109/00498254.2014.941963. Epub 2014 Jul 17. Xenobiotica. 2014. PMID: 25030041
-
Are endosomal trafficking parameters better targets for improving mAb pharmacokinetics than FcRn binding affinity?Mol Immunol. 2013 Dec;56(4):660-74. doi: 10.1016/j.molimm.2013.05.008. Epub 2013 Aug 2. Mol Immunol. 2013. PMID: 23917469 Review.
-
Application of FcRn binding assays to guide mAb development.Drug Metab Dispos. 2014 Nov;42(11):1867-72. doi: 10.1124/dmd.114.059089. Epub 2014 Jul 14. Drug Metab Dispos. 2014. PMID: 25024401 Review.
Cited by
-
Physiologically-based modeling of monoclonal antibody pharmacokinetics in drug discovery and development.Drug Metab Pharmacokinet. 2019 Feb;34(1):3-13. doi: 10.1016/j.dmpk.2018.11.002. Epub 2018 Nov 22. Drug Metab Pharmacokinet. 2019. PMID: 30522890 Free PMC article. Review.
-
Pharmacokinetic and Pharmacodynamic Properties of Drug Delivery Systems.J Pharmacol Exp Ther. 2019 Sep;370(3):570-580. doi: 10.1124/jpet.119.257113. Epub 2019 Mar 5. J Pharmacol Exp Ther. 2019. PMID: 30837281 Free PMC article. Review.
-
Mathematical Models to Characterize the Absorption, Distribution, Metabolism, and Excretion of Protein Therapeutics.Drug Metab Dispos. 2022 Jun;50(6):867-878. doi: 10.1124/dmd.121.000460. Epub 2022 Feb 23. Drug Metab Dispos. 2022. PMID: 35197311 Free PMC article. Review.
-
Heparin chromatography as an in vitro predictor for antibody clearance rate through pinocytosis.MAbs. 2020 Jan-Dec;12(1):1683432. doi: 10.1080/19420862.2019.1683432. MAbs. 2020. PMID: 31769731 Free PMC article.
-
Model-Based Assessment of the Contribution of Monocytes and Macrophages to the Pharmacokinetics of Monoclonal Antibodies.Pharm Res. 2022 Feb;39(2):239-250. doi: 10.1007/s11095-022-03177-2. Epub 2022 Feb 3. Pharm Res. 2022. PMID: 35118567
References
-
- Bittner B, Schmidt J. Subcutaneous administration of monoclonal antibodies in oncology as alternative to established intravenous infusion. Pharm Ind. 2012;74:638–43.
-
- McDonald TA, Zepeda ML, Tomlinson MJ, Bee WH, Ivens IA. Subcutaneous administration of biotherapeutics: current experience in animal models. Curr Opin Mol Ther. 2010;12(4):461–70. PMID:20677097. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
