Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS
- PMID: 29618656
- PMCID: PMC5928858
- DOI: 10.1172/jci.insight.99405
Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS
Abstract
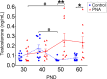
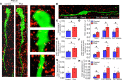
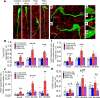
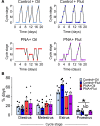
Androgen excess is a hallmark of polycystic ovary syndrome (PCOS), a prevalent yet poorly understood endocrine disorder. Evidence from women and preclinical animal models suggests that elevated perinatal androgens can elicit PCOS onset in adulthood, implying androgen actions in both PCOS ontogeny and adult pathophysiology. Prenatally androgenized (PNA) mice exhibit a robust increase of progesterone-sensitive GABAergic inputs to gonadotropin-releasing hormone (GnRH) neurons implicated in the pathogenesis of PCOS. It is unclear when altered GABAergic wiring develops in the brain, and whether these central abnormalities are dependent upon adult androgen excess. Using GnRH-GFP-transgenic mice, we determined that increased GABA input to GnRH neurons occurs prior to androgen excess and the manifestation of reproductive impairments in PNA mice. These data suggest that brain circuit abnormalities precede the postpubertal development of PCOS traits. Despite the apparent developmental programming of circuit abnormalities, long-term blockade of androgen receptor signaling from early adulthood rescued normal GABAergic wiring onto GnRH neurons, improved ovarian morphology, and restored reproductive cycles in PNA mice. Therefore, androgen excess maintains changes in female brain wiring linked to PCOS features and the blockade of androgen receptor signaling reverses both the central and peripheral PNA-induced PCOS phenotype.
Keywords: Endocrinology; Fertility; Neuroendocrine regulation; Neuroscience; Sex hormones.
Conflict of interest statement
Figures


Similar articles
-
Investigating GABA Neuron-Specific Androgen Receptor Knockout in two Hyperandrogenic Models of PCOS.Endocrinology. 2024 May 27;165(7):bqae060. doi: 10.1210/endocr/bqae060. Endocrinology. 2024. PMID: 38788194 Free PMC article.
-
Prepubertal Development of GABAergic Transmission to Gonadotropin-Releasing Hormone (GnRH) Neurons and Postsynaptic Response Are Altered by Prenatal Androgenization.J Neurosci. 2018 Feb 28;38(9):2283-2293. doi: 10.1523/JNEUROSCI.2304-17.2018. Epub 2018 Jan 26. J Neurosci. 2018. PMID: 29374136 Free PMC article.
-
Enhancement of a robust arcuate GABAergic input to gonadotropin-releasing hormone neurons in a model of polycystic ovarian syndrome.Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):596-601. doi: 10.1073/pnas.1415038112. Epub 2014 Dec 30. Proc Natl Acad Sci U S A. 2015. PMID: 25550522 Free PMC article.
-
The neuroendocrine genesis of polycystic ovary syndrome: A role for arcuate nucleus GABA neurons.J Steroid Biochem Mol Biol. 2016 Jun;160:106-17. doi: 10.1016/j.jsbmb.2015.10.002. Epub 2015 Oct 9. J Steroid Biochem Mol Biol. 2016. PMID: 26455490 Review.
-
Polycystic ovary syndrome: Understanding the role of the brain.Front Neuroendocrinol. 2017 Jul;46:1-14. doi: 10.1016/j.yfrne.2017.05.002. Epub 2017 May 25. Front Neuroendocrinol. 2017. PMID: 28551304 Review.
Cited by
-
Deletion of Androgen Receptor in LepRb Cells Improves Estrous Cycles in Prenatally Androgenized Mice.Endocrinology. 2023 Jan 9;164(3):bqad015. doi: 10.1210/endocr/bqad015. Endocrinology. 2023. PMID: 36683455 Free PMC article.
-
The Role of the Brain in the Pathogenesis and Physiology of Polycystic Ovary Syndrome (PCOS).Med Sci (Basel). 2019 Aug 2;7(8):84. doi: 10.3390/medsci7080084. Med Sci (Basel). 2019. PMID: 31382541 Free PMC article. Review.
-
The influence of maternal androgen excess on the male reproductive axis.Sci Rep. 2019 Dec 11;9(1):18908. doi: 10.1038/s41598-019-55436-9. Sci Rep. 2019. PMID: 31827225 Free PMC article.
-
Potential for NPY receptor-related therapies for polycystic ovary syndrome: an updated review.Hormones (Athens). 2023 Sep;22(3):441-451. doi: 10.1007/s42000-023-00460-8. Epub 2023 Jul 14. Hormones (Athens). 2023. PMID: 37452264 Free PMC article. Review.
-
Animal Models for Human Polycystic Ovary Syndrome (PCOS) Focused on the Use of Indirect Hormonal Perturbations: A Review of the Literature.Int J Mol Sci. 2019 Jun 3;20(11):2720. doi: 10.3390/ijms20112720. Int J Mol Sci. 2019. PMID: 31163591 Free PMC article. Review.
References
-
- Pastor CL, Griffin-Korf ML, Aloi JA, Evans WS, Marshall JC. Polycystic ovary syndrome: evidence for reduced sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 1998;83(2):582–590. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

