Immune Toxicity with Checkpoint Inhibition for Metastatic Melanoma: Case Series and Clinical Management
- PMID: 29610684
- PMCID: PMC5828308
- DOI: 10.1155/2018/9602540
Immune Toxicity with Checkpoint Inhibition for Metastatic Melanoma: Case Series and Clinical Management
Abstract
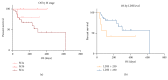
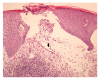
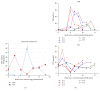
Immune checkpoint inhibitors (anti-PD-1 and anti-CTLA-4 antibodies) are a standard of care for advanced melanoma. Novel toxicities comprise immune-related adverse events (irAE). With increasing use, irAE require recognition, practical management strategies, and multidisciplinary care. We retrospectively evaluated the incidence, kinetics, and management of irAE in 41 patients receiving anti-PD-1 antibody therapy (pembrolizumab) for advanced melanoma. 63% received prior anti-CTLA-4 antibody therapy (ipilimumab). IrAE occurred in 54%, most commonly dermatological (24%), rheumatological (22%), and thyroid dysfunction (12%). Thyroiditis was characterised by a brief asymptomatic hyperthyroid phase followed by a symptomatic hypothyroid phase requiring thyroxine replacement. Transplant rejection doses of methylprednisolone were necessary to manage refractory hepatotoxicity. A bullous pemphigoid-like skin reaction with refractory pruritus responded to corticosteroids and neuropathic analgesia. Disabling grade 3-4 oligoarthritis required sulfasalazine therapy in combination with steroids. The median interval between the last dose of anti-CTLA-4 antibody and the first dose of anti-PD-1 therapy was 2.0 months (range: 0.4 to 22.4). Toxicities may occur late; this requires vigilance and multidisciplinary management which may allow effective anticancer therapy to continue. Management algorithms for thyroiditis, hypophysitis, arthralgia/arthritis, colitis, steroid-refractory hepatitis, and skin toxicity are discussed.
Figures
Similar articles
-
Management of toxicities of immune checkpoint inhibitors.Cancer Treat Rev. 2016 Mar;44:51-60. doi: 10.1016/j.ctrv.2016.02.001. Epub 2016 Feb 6. Cancer Treat Rev. 2016. PMID: 26874776 Review.
-
Thrombocytopenia in patients with melanoma receiving immune checkpoint inhibitor therapy.J Immunother Cancer. 2017 Feb 21;5:8. doi: 10.1186/s40425-017-0210-0. eCollection 2017. J Immunother Cancer. 2017. PMID: 28239462 Free PMC article.
-
Ipilimumab-induced toxicities and the gastroenterologist.J Gastroenterol Hepatol. 2015 Apr;30(4):657-66. doi: 10.1111/jgh.12888. J Gastroenterol Hepatol. 2015. PMID: 25641691 Review.
-
Management of side effects of immune checkpoint blockade by anti-CTLA-4 and anti-PD-1 antibodies in metastatic melanoma.J Dtsch Dermatol Ges. 2016 Jul;14(7):662-81. doi: 10.1111/ddg.13047. J Dtsch Dermatol Ges. 2016. PMID: 27373241
-
Standard-dose pembrolizumab in combination with reduced-dose ipilimumab for patients with advanced melanoma (KEYNOTE-029): an open-label, phase 1b trial.Lancet Oncol. 2017 Sep;18(9):1202-1210. doi: 10.1016/S1470-2045(17)30428-X. Epub 2017 Jul 17. Lancet Oncol. 2017. PMID: 28729151 Clinical Trial.
Cited by
-
EULAR points to consider for the diagnosis and management of rheumatic immune-related adverse events due to cancer immunotherapy with checkpoint inhibitors.Ann Rheum Dis. 2021 Jan;80(1):36-48. doi: 10.1136/annrheumdis-2020-217139. Epub 2020 Apr 23. Ann Rheum Dis. 2021. PMID: 32327425 Free PMC article.
-
Thyroid dysfunction and risk of cutaneous malignant melanoma: a bidirectional two-sample Mendelian randomization study.Front Endocrinol (Lausanne). 2023 Nov 29;14:1239883. doi: 10.3389/fendo.2023.1239883. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 38093968 Free PMC article.
-
Epidemiology and risk factors for the development of cutaneous toxicities in patients treated with immune-checkpoint inhibitors: A United States population-level analysis.J Am Acad Dermatol. 2022 Mar;86(3):563-572. doi: 10.1016/j.jaad.2021.03.094. Epub 2021 Apr 2. J Am Acad Dermatol. 2022. PMID: 33819538 Free PMC article.
-
Management of the Adverse Effects of Immune Checkpoint Inhibitors.Vaccines (Basel). 2020 Oct 1;8(4):575. doi: 10.3390/vaccines8040575. Vaccines (Basel). 2020. PMID: 33019641 Free PMC article. Review.
-
Campylobacteriosis following immunosuppression for immune checkpoint inhibitor-related toxicity.J Immunother Cancer. 2020 Oct;8(2):e000577. doi: 10.1136/jitc-2020-000577. J Immunother Cancer. 2020. PMID: 33020237 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

