Cytochrome P450 3A4 Induction: Lumacaftor versus Ivacaftor Potentially Resulting in Significantly Reduced Plasma Concentration of Ivacaftor
- PMID: 29595119
- PMCID: PMC6350194
- DOI: 10.2174/1872312812666180328105259
Cytochrome P450 3A4 Induction: Lumacaftor versus Ivacaftor Potentially Resulting in Significantly Reduced Plasma Concentration of Ivacaftor
Abstract
Background & objective: Since the release of ivacaftor-lumacaftor, several red-flags have been raised that highlight the clinical efficacy of this combination strategy that may be limited due to antagonistic drug-drug interactions.
Method: The effect of ivacaftor, its major metabolites M1 and M6, lumacaftor and the novel cystic fibrosis transmembrane conductance regulator (CFTR) modulator tezacaftor at 10 µg/mL on the enzymatic activity of the major xenobiotic metabolizing enzymes CYP1A2 and CYP3A4 as well as the minor enzymes CYP2B6 and CYP2C9 was assayed.
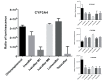
Results: Lumacaftor (3.74 x 105 ± 3.11 x 104 RLU), and ivacaftor-M6 (3.43 x 105 ± 7.61 x 103 RLU) markedly induced the activity of CYP3A4. Ivacaftor (2.22 x 105 ± 3.94 x 104 RLU) showed a lower relative ratio of luminescence units compared to chloramphenicol (3.17 x 105 ± 1.55 x 104 RLU). Interestingly, ivacaftor-M1 (6.74 x 104 ± 3.09 x 104 RLU) and the novel CFTR modulator tezacaftor (2.40 x 104 ± 8.14 x 104 RLU) did not show CYP3A4 induction. In the CYP1A2 and CYP2C9 assay, all metabolites showed a decrease in the ratio of luminescence units compared to the controls. Ivacaftor, its major metabolites, lumacaftor and tezacaftor all showed a slight increase in the ratio of luminescence units compared to the control rifampin with CYP2B6.
Conclusion: All in all, present findings would suggest that lumacaftor and ivacaftor-M6 are strong inducers of CYP3A4, potentially reducing ivacaftor concentrations; ivacaftor itself induces CYP3A4 to some extent.
Keywords: CFTR modulator; Cystic fibrosis; cytochrome; drug interactions; ivacaftor; lumacaftor..
Copyright© Bentham Science Publishers; For any queries, please email at epub@benthamscience.org.
Figures
Similar articles
-
Can Cystic Fibrosis Patients Finally Catch a Breath With Lumacaftor/Ivacaftor?Clin Pharmacol Ther. 2017 Jan;101(1):130-141. doi: 10.1002/cpt.548. Epub 2016 Nov 23. Clin Pharmacol Ther. 2017. PMID: 27804127 Free PMC article. Review.
-
Lumacaftor alone and combined with ivacaftor: preclinical and clinical trial experience of F508del CFTR correction.Expert Rev Respir Med. 2016;10(1):5-17. doi: 10.1586/17476348.2016.1122527. Epub 2015 Dec 9. Expert Rev Respir Med. 2016. PMID: 26581802
-
Metabolomic responses to lumacaftor/ivacaftor in cystic fibrosis.Pediatr Pulmonol. 2018 May;53(5):583-591. doi: 10.1002/ppul.23972. Epub 2018 Feb 20. Pediatr Pulmonol. 2018. PMID: 29461009
-
The safety of lumacaftor and ivacaftor for the treatment of cystic fibrosis.Expert Opin Drug Saf. 2017 Nov;16(11):1305-1311. doi: 10.1080/14740338.2017.1372419. Epub 2017 Sep 21. Expert Opin Drug Saf. 2017. PMID: 28846049 Free PMC article. Review.
-
Lumacaftor-ivacaftor in the treatment of cystic fibrosis: design, development and place in therapy.Drug Des Devel Ther. 2019 Jul 19;13:2405-2412. doi: 10.2147/DDDT.S153719. eCollection 2019. Drug Des Devel Ther. 2019. PMID: 31409974 Free PMC article. Review.
Cited by
-
Contraceptive use among women with cystic fibrosis: A pilot study linking reproductive health questions to the Cystic Fibrosis Foundation National Patient Registry.Contraception. 2020 Jun;101(6):420-426. doi: 10.1016/j.contraception.2020.02.006. Epub 2020 Feb 26. Contraception. 2020. PMID: 32109430 Free PMC article.
-
Hydrocodone, Oxycodone, and Morphine Metabolism and Drug-Drug Interactions.J Pharmacol Exp Ther. 2023 Nov;387(2):150-169. doi: 10.1124/jpet.123.001651. Epub 2023 Sep 7. J Pharmacol Exp Ther. 2023. PMID: 37679047 Free PMC article. Review.
-
New Therapies to Correct the Cystic Fibrosis Basic Defect.Int J Mol Sci. 2021 Jun 8;22(12):6193. doi: 10.3390/ijms22126193. Int J Mol Sci. 2021. PMID: 34201249 Free PMC article. Review.
-
Treatment of Polarized Cystic Fibrosis Airway Cells With HGF Prevents VX-661-Rescued F508del-CFTR Destabilization Caused by Prolonged Co-exposure to VX-770.Front Mol Biosci. 2021 Dec 22;8:812101. doi: 10.3389/fmolb.2021.812101. eCollection 2021. Front Mol Biosci. 2021. PMID: 35004859 Free PMC article.
-
The Intestinal Microbiome and Cystic Fibrosis Transmembrane Conductance Regulator Modulators: Emerging Themes in the Management of Gastrointestinal Manifestations of Cystic Fibrosis.Curr Gastroenterol Rep. 2021 Aug 27;23(10):17. doi: 10.1007/s11894-021-00817-2. Curr Gastroenterol Rep. 2021. PMID: 34448955 Review.
References
-
- Bobadilla J.L., Macek M., Jr, Fine J.P., Farrell P.M. Cystic fibrosis: A worldwide analysis of CFTR mutations-correlation with incidence data and application to screening. Hum. Mutat. 2002;19(6):75–606. - PubMed
-
- Ramsey B.W., Davies J., McElvaney N.G., Tullis E., Bell S.C., Drevinek P., Griese M., McKone E.F., Wainwright C.E., Konstan M.W., Moss R., Ratjen F., Sermet-Gaudelus I., Rowe S.M., Dong Q., Rodriguez S., Yen K., Ordonez C., Elborn J.S.A. CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011;365(18):1663–1672. - PMC - PubMed
-
- Wainwright C.E., Elborn J.S., Ramsey B.W., Marigowda G., Huang X., Cipolli M., Colombo C., Davies J.C., De Boeck K., Flume P.A., Konstan M.W., McColley S.A., McCoy K., McKone E.F., Munck A., Ratjen F., Rowe S.M., Waltz D., Boyle M.P. Lumacaftor-Ivacaftor in Patients with Cystic Fibrosis Homozygous for Phe508del CFTR. N. Engl. J. Med. 2015;373(3):220–231. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

