Intestinal epithelial cell polarity defects in disease: lessons from microvillus inclusion disease
- PMID: 29590640
- PMCID: PMC5894939
- DOI: 10.1242/dmm.031088
Intestinal epithelial cell polarity defects in disease: lessons from microvillus inclusion disease
Abstract
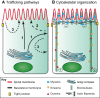
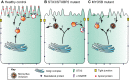
The intestinal epithelium is a highly organized tissue. The establishment of epithelial cell polarity, with distinct apical and basolateral plasma membrane domains, is pivotal for both barrier formation and for the uptake and vectorial transport of nutrients. The establishment of cell polarity requires a specialized subcellular machinery to transport and recycle proteins to their appropriate location. In order to understand and treat polarity-associated diseases, it is necessary to understand epithelial cell-specific trafficking mechanisms. In this Review, we focus on cell polarity in the adult mammalian intestine. We discuss how intestinal epithelial polarity is established and maintained, and how disturbances in the trafficking machinery can lead to a polarity-associated disorder, microvillus inclusion disease (MVID). Furthermore, we discuss the recent developments in studying MVID, including the creation of genetically manipulated cell lines, mouse models and intestinal organoids, and their uses in basic and applied research.
Keywords: Epithelial cells; Intestine; Microvillus inclusion disease; Polarity.
© 2018. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures

Similar articles
-
An inducible mouse model for microvillus inclusion disease reveals a role for myosin Vb in apical and basolateral trafficking.Proc Natl Acad Sci U S A. 2015 Oct 6;112(40):12408-13. doi: 10.1073/pnas.1516672112. Epub 2015 Sep 21. Proc Natl Acad Sci U S A. 2015. PMID: 26392529 Free PMC article.
-
Dynamic Formation of Microvillus Inclusions During Enterocyte Differentiation in Munc18-2-Deficient Intestinal Organoids.Cell Mol Gastroenterol Hepatol. 2018 Aug 14;6(4):477-493.e1. doi: 10.1016/j.jcmgh.2018.08.001. eCollection 2018. Cell Mol Gastroenterol Hepatol. 2018. PMID: 30364784 Free PMC article.
-
The localisation of the apical Par/Cdc42 polarity module is specifically affected in microvillus inclusion disease.Biol Cell. 2016 Jan;108(1):19-28. doi: 10.1111/boc.201500034. Epub 2015 Dec 8. Biol Cell. 2016. PMID: 26526116
-
Recent advances in understanding and managing malabsorption: focus on microvillus inclusion disease.F1000Res. 2019 Dec 5;8:F1000 Faculty Rev-2061. doi: 10.12688/f1000research.20762.1. eCollection 2019. F1000Res. 2019. PMID: 31824659 Free PMC article. Review.
-
Uncovering the Relationship Between Genes and Phenotypes Beyond the Gut in Microvillus Inclusion Disease.Cell Mol Gastroenterol Hepatol. 2024;17(6):983-1005. doi: 10.1016/j.jcmgh.2024.01.015. Epub 2024 Feb 1. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38307491 Free PMC article. Review.
Cited by
-
Applications of human organoids in the personalized treatment for digestive diseases.Signal Transduct Target Ther. 2022 Sep 27;7(1):336. doi: 10.1038/s41392-022-01194-6. Signal Transduct Target Ther. 2022. PMID: 36167824 Free PMC article. Review.
-
Downregulation of V-ATPase V0 Sector Induces Microvillus Atrophy Independently of Apical Trafficking in the Mammalian Intestine.Cell Mol Gastroenterol Hepatol. 2024;17(6):1072-1075. doi: 10.1016/j.jcmgh.2024.02.011. Epub 2024 Feb 16. Cell Mol Gastroenterol Hepatol. 2024. PMID: 38369130 Free PMC article. No abstract available.
-
Butyrate Glycerides Protect against Intestinal Inflammation and Barrier Dysfunction in Mice.Nutrients. 2022 Sep 26;14(19):3991. doi: 10.3390/nu14193991. Nutrients. 2022. PMID: 36235644 Free PMC article.
-
Tight Junction Proteins Join the Local Force for Bulk Endocytosis and Microvillus Inclusion.Cell Mol Gastroenterol Hepatol. 2021;12(1):348-349. doi: 10.1016/j.jcmgh.2021.02.012. Epub 2021 Mar 20. Cell Mol Gastroenterol Hepatol. 2021. PMID: 33757764 Free PMC article. No abstract available.
-
Current understanding of the gut microbiota shaping mechanisms.J Biomed Sci. 2019 Aug 21;26(1):59. doi: 10.1186/s12929-019-0554-5. J Biomed Sci. 2019. PMID: 31434568 Free PMC article. Review.
References
-
- Antileo E., Garri C., Tapia V., Muñoz J. P., Chiong M., Nualart F., Lavandero S., Fernández J. and Núñez M. T. (2013). Endocytic pathway of exogenous iron-loaded ferritin in intestinal epithelial (Caco-2) cells. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G655-G661. 10.1152/ajpgi.00472.2012 - DOI - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

