Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Pre- and Posttreatment Breast Cancers in the SWOG S0800 Phase II Neoadjuvant Chemotherapy Trial
- PMID: 29588392
- PMCID: PMC6548451
- DOI: 10.1158/1535-7163.MCT-17-1005
Tumor-Infiltrating Lymphocytes and PD-L1 Expression in Pre- and Posttreatment Breast Cancers in the SWOG S0800 Phase II Neoadjuvant Chemotherapy Trial
Abstract
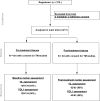
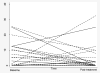
Our aim was to examine the association of pretreatment tumor-infiltrating lymphocyte (TIL) count and PD-L1 levels with pathologic complete response (pCR) and assess immune marker changes following treatment in tumor specimens from the S0800 clinical trial, which randomized patients to bevacizumab + nab-paclitaxel, followed by doxorubicin/cyclophosphamide (AC) versus two control arms without bevacizumab (varying sequence of AC and nab-paclitaxel). TILs were assessed in 124 pre- and 62 posttreatment tissues (including 59 pairs). PD-L1 was assessed in 120 pre- and 43 posttreatment tissues (including 39 pairs) using the 22C3 antibody. Baseline and treatment-induced immune changes were correlated with pCR and survival using estrogen receptor (ER) and treatment-adjusted logistic and Cox regressions, respectively. At baseline, the mean TIL count was 17.4% (17% had zero TILs, 9% had ≥50% TILs). Posttreatment, mean TIL count decreased to 11% (5% had no TILs, 2% had >50% TILs). In paired samples, the mean TIL change was 15% decrease. Baseline PD-L1 was detected in 43% of cases (n = 5 in tumor cells, n = 29 stroma, n = 18 tumor + stroma). Posttreatment, PD-L1 expression was not significantly lower (33%). Higher baseline TIL count and PD-L1 positivity rate were associated with higher pCR rate even after adjustment for treatment and ER status (P = 0.018). There was no association between TIL counts, PD-L1 expression, and survival due to few events. In conclusion, TIL counts, but not PD-L1 expression, decreased significantly after treatment. Continued PD-L1 expression in some residual cancers raises the possibility that adjuvant immune checkpoint inhibitor therapy could improve survival in this patient population. Mol Cancer Ther; 17(6); 1324-31. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Figures


Similar articles
-
Effect of neoadjuvant chemotherapy on tumor-infiltrating lymphocytes and PD-L1 expression in breast cancer and its clinical significance.Breast Cancer Res. 2017 Aug 7;19(1):91. doi: 10.1186/s13058-017-0884-8. Breast Cancer Res. 2017. PMID: 28784153 Free PMC article.
-
Immune profiling of pre- and post-treatment breast cancer tissues from the SWOG S0800 neoadjuvant trial.J Immunother Cancer. 2019 Apr 10;7(1):88. doi: 10.1186/s40425-019-0563-7. J Immunother Cancer. 2019. PMID: 30967156 Free PMC article.
-
The role of programmed death ligand-1 and tumor-infiltrating lymphocytes in breast cancer overexpressing HER2 gene.Breast Cancer Res Treat. 2018 Jul;170(2):293-302. doi: 10.1007/s10549-018-4745-7. Epub 2018 Mar 9. Breast Cancer Res Treat. 2018. PMID: 29524062 Free PMC article.
-
Significance of evaluating tumor-infiltrating lymphocytes (TILs) and programmed cell death-ligand 1 (PD-L1) expression in breast cancer.Med Mol Morphol. 2017 Dec;50(4):185-194. doi: 10.1007/s00795-017-0170-y. Epub 2017 Sep 21. Med Mol Morphol. 2017. PMID: 28936553 Review.
-
A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients.Breast Cancer Res Treat. 2019 Jan;173(2):255-266. doi: 10.1007/s10549-018-4981-x. Epub 2018 Oct 15. Breast Cancer Res Treat. 2019. PMID: 30324273 Review.
Cited by
-
PD-L1 Protein Expression on Both Tumor Cells and Macrophages are Associated with Response to Neoadjuvant Durvalumab with Chemotherapy in Triple-negative Breast Cancer.Clin Cancer Res. 2020 Oct 15;26(20):5456-5461. doi: 10.1158/1078-0432.CCR-20-1303. Epub 2020 Jul 24. Clin Cancer Res. 2020. PMID: 32709714 Free PMC article. Clinical Trial.
-
G Protein-Coupled Estrogen Receptor in Immune Cells and Its Role in Immune-Related Diseases.Front Endocrinol (Lausanne). 2020 Oct 2;11:579420. doi: 10.3389/fendo.2020.579420. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33133022 Free PMC article. Review.
-
Immune microenvironment changes induced by neoadjuvant chemotherapy in triple-negative breast cancers: the MIMOSA-1 study.Breast Cancer Res. 2021 May 26;23(1):61. doi: 10.1186/s13058-021-01437-4. Breast Cancer Res. 2021. PMID: 34039396 Free PMC article.
-
Association of B7-H4, PD-L1, and tumor infiltrating lymphocytes with outcomes in breast cancer.NPJ Breast Cancer. 2018 Dec 10;4:40. doi: 10.1038/s41523-018-0095-1. eCollection 2018. NPJ Breast Cancer. 2018. PMID: 30564631 Free PMC article.
-
Transcriptomic Properties of HER2+ Ductal Carcinoma In Situ of the Breast Associate with Absence of Immune Cells.Biology (Basel). 2021 Aug 12;10(8):768. doi: 10.3390/biology10080768. Biology (Basel). 2021. PMID: 34440000 Free PMC article.
References
-
- Mougalian SS, Soulos PR, Killelea BK, Lannin DR, Abu-Khalaf MM, DiGiovanna MP, et al. Use of neoadjuvant chemotherapy for patients with stage I to III breast cancer in the United States. Cancer. 2015;121:2544–52. - PubMed
-
- Killelea BK, Yang VQ, Mougalian S, Horowitz NR, Pusztai L, Chagpar AB, et al. Neoadjuvant chemotherapy for breast cancer increases the rate of breast conservation: results from the National Cancer Database. J Am Coll Surg. 2015;220:1063–9. - PubMed
-
- Symmans WF, Peintinger F, Hatzis C, Rajan R, Kuerer H, Valero V, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2007;25:4414–22. - PubMed
-
- Toi ML-J, Lee ES, Ohtani S, Im Y-H, Im S-A, Park B-W, et al. A phase III trial of adjuvant capecitabine in breast cancer patients with HER2-negative pathologic residual invasive disease after neoadjuvant chemotherapy (CREATE-X, JBCRG-04); Proceedings of the Thirty-Eighth Annual CTRC-AACR San Antonio Breast Cancer Symposium; 2015 Dec 8-12; San Antonio. TX Philadelphia (PA): AACR; 2015. 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

