Regulation of alveolar macrophage death in acute lung inflammation
- PMID: 29587748
- PMCID: PMC5872399
- DOI: 10.1186/s12931-018-0756-5
Regulation of alveolar macrophage death in acute lung inflammation
Abstract
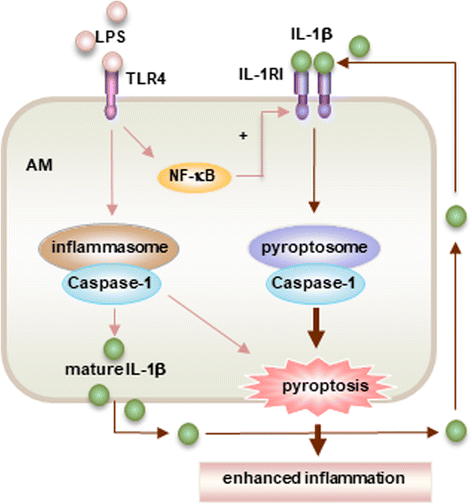
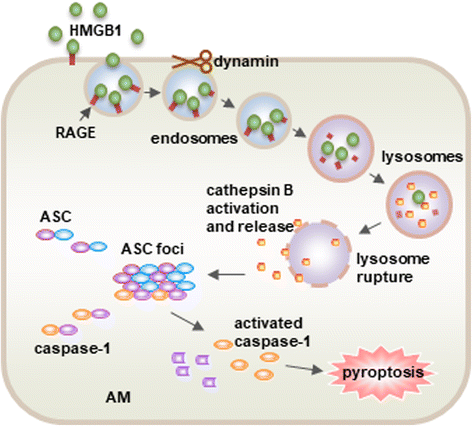
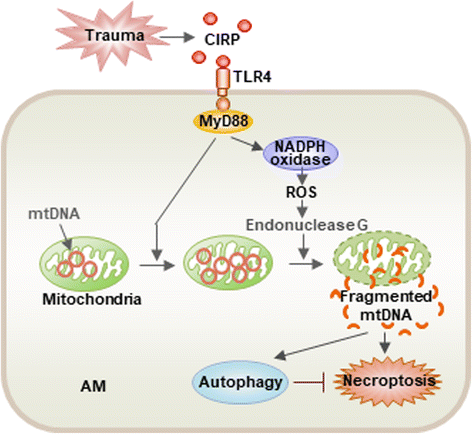
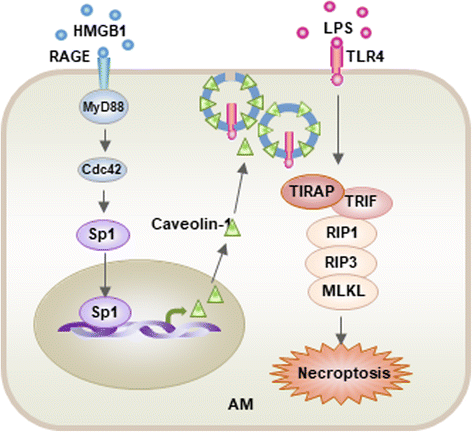
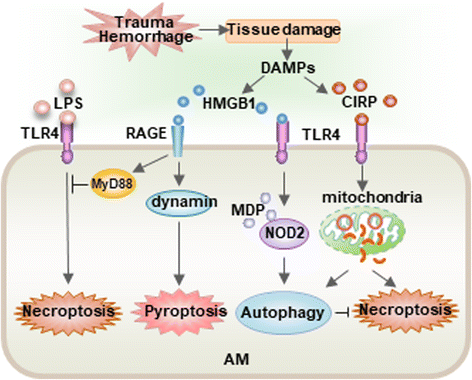
Acute lung injury (ALI) and its severe form, known as acute respiratory distress syndrome (ARDS), are caused by direct pulmonary insults and indirect systemic inflammatory responses that result from conditions such as sepsis, trauma, and major surgery. The reciprocal influences between pulmonary and systemic inflammation augments the inflammatory process in the lung and promotes the development of ALI. Emerging evidence has revealed that alveolar macrophage (AM) death plays important roles in the progression of lung inflammation through its influence on other immune cell populations in the lung. Cell death and tissue inflammation form a positive feedback cycle, ultimately leading to exaggerated inflammation and development of disease. Pharmacological manipulation of AM death signals may serve as a logical therapeutic strategy for ALI/ARDS. This review will focus on recent advances in the regulation and underlying mechanisms of AM death as well as the influence of AM death on the development of ALI.
Keywords: Acute lung injury; Autophagy; Cell death; Macrophages; Necroptosis; Pyroptosis.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Comparative analysis of the alveolar macrophage proteome in ALI/ARDS patients between the exudative phase and recovery phase.BMC Immunol. 2013 Jun 17;14:25. doi: 10.1186/1471-2172-14-25. BMC Immunol. 2013. PMID: 23773529 Free PMC article.
-
Extracellular histones aggravate inflammation in ARDS by promoting alveolar macrophage pyroptosis.Mol Immunol. 2021 Jul;135:53-61. doi: 10.1016/j.molimm.2021.04.002. Epub 2021 Apr 16. Mol Immunol. 2021. PMID: 33873094
-
Alpha-linolenic acid pretreatment alleviates NETs-induced alveolar macrophage pyroptosis by inhibiting pyrin inflammasome activation in a mouse model of sepsis-induced ALI/ARDS.Front Immunol. 2023 Mar 27;14:1146612. doi: 10.3389/fimmu.2023.1146612. eCollection 2023. Front Immunol. 2023. PMID: 37051243 Free PMC article.
-
The Role of Deubiquitinating Enzymes in Acute Lung Injury and Acute Respiratory Distress Syndrome.Int J Mol Sci. 2020 Jul 8;21(14):4842. doi: 10.3390/ijms21144842. Int J Mol Sci. 2020. PMID: 32650621 Free PMC article. Review.
-
Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms.Pharmacol Res. 2021 Jan;163:105224. doi: 10.1016/j.phrs.2020.105224. Epub 2020 Sep 29. Pharmacol Res. 2021. PMID: 33007416 Free PMC article. Review.
Cited by
-
The effect of environmental diesel exhaust pollution on SARS-CoV-2 infection: The mechanism of pulmonary ground glass opacity.Environ Toxicol Pharmacol. 2021 Aug;86:103657. doi: 10.1016/j.etap.2021.103657. Epub 2021 Apr 7. Environ Toxicol Pharmacol. 2021. PMID: 33838330 Free PMC article. Review.
-
The severe COVID-19: A sepsis induced by viral infection? And its immunomodulatory therapy.Chin J Traumatol. 2020 Aug;23(4):190-195. doi: 10.1016/j.cjtee.2020.06.002. Epub 2020 Jun 15. Chin J Traumatol. 2020. PMID: 32690231 Free PMC article. Review.
-
Acute Endotoxemia-Induced Respiratory and Intestinal Dysbiosis.Int J Mol Sci. 2022 Oct 1;23(19):11602. doi: 10.3390/ijms231911602. Int J Mol Sci. 2022. PMID: 36232913 Free PMC article.
-
Xanthoxylin Attenuates Lipopolysaccharide-Induced Lung Injury through Modulation of Akt/HIF-1α/NF-κB and Nrf2 Pathways.Int J Mol Sci. 2024 Aug 10;25(16):8742. doi: 10.3390/ijms25168742. Int J Mol Sci. 2024. PMID: 39201430 Free PMC article.
-
Preventing the development of severe COVID-19 by modifying immunothrombosis.Life Sci. 2021 Jan 1;264:118617. doi: 10.1016/j.lfs.2020.118617. Epub 2020 Oct 20. Life Sci. 2021. PMID: 33096114 Free PMC article. Review.
References
-
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

