Neutrophil-Based Drug Delivery Systems
- PMID: 29577477
- PMCID: PMC6161715
- DOI: 10.1002/adma.201706245
Neutrophil-Based Drug Delivery Systems
Abstract
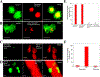
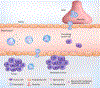
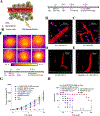
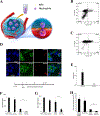
White blood cells (WBCs) are a major component of immunity in response to pathogen invasion. Neutrophils are the most abundant WBCs in humans, playing a central role in acute inflammation induced by pathogens. Adhesion to vasculature and tissue infiltration of neutrophils are key processes in acute inflammation. Many inflammatory/autoimmune disorders and cancer therapies have been found to be involved in activation and tissue infiltration of neutrophils. A promising strategy to develop novel targeted drug delivery systems is the targeting and exploitation of activated neutrophils. Herein, a new drug delivery platform based on neutrophils is reviewed. There are two types of drug delivery systems: neutrophils as carriers and neutrophil-membrane-derived nanovesicles. It is discussed how nanoparticles hijack neutrophils in vivo to deliver therapeutics across blood vessel barriers and how neutrophil-membrane-derived nanovesicles target inflamed vasculature. Finally, the potential applications of neutrophil-based drug delivery systems in treating inflammation and cancers are presented.
Keywords: cancer; inflammation; nanovesicles; neutrophils; targeted drug delivery.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Conflict of interest statement
Conflicts of Interest
The authors declare no conflicts of interest.
Figures




Similar articles
-
Recent progress in therapeutic strategies and biomimetic nanomedicines based on neutrophils for inflammation treatment.Nanomedicine (Lond). 2023 Feb;18(5):485-500. doi: 10.2217/nnm-2022-0211. Epub 2023 May 11. Nanomedicine (Lond). 2023. PMID: 37165973 Review.
-
Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation To Protect Mouse Brain Injury from Ischemic Stroke.ACS Nano. 2019 Feb 26;13(2):1272-1283. doi: 10.1021/acsnano.8b06572. Epub 2019 Jan 28. ACS Nano. 2019. PMID: 30673266 Free PMC article.
-
Neutrophil-Mediated Delivery of Therapeutic Nanoparticles across Blood Vessel Barrier for Treatment of Inflammation and Infection.ACS Nano. 2015 Dec 22;9(12):11800-11. doi: 10.1021/acsnano.5b05583. Epub 2015 Nov 5. ACS Nano. 2015. PMID: 26516654 Free PMC article.
-
Neutrophil mediated drug delivery for targeted glioblastoma therapy: A comprehensive review.Biomed Pharmacother. 2022 Dec;156:113841. doi: 10.1016/j.biopha.2022.113841. Epub 2022 Nov 1. Biomed Pharmacother. 2022. PMID: 36411657 Review.
-
Cell membrane-formed nanovesicles for disease-targeted delivery.J Control Release. 2016 Feb 28;224:208-216. doi: 10.1016/j.jconrel.2016.01.024. Epub 2016 Jan 14. J Control Release. 2016. PMID: 26778696 Free PMC article.
Cited by
-
Advances in drug delivery systems utilizing blood cells and their membrane-derived microvesicles.Drug Deliv. 2024 Dec;31(1):2425156. doi: 10.1080/10717544.2024.2425156. Epub 2024 Nov 8. Drug Deliv. 2024. PMID: 39520082 Free PMC article. Review.
-
Exosome-based cell therapy for diabetic foot ulcers: Present and prospect.Heliyon. 2024 Oct 11;10(20):e39251. doi: 10.1016/j.heliyon.2024.e39251. eCollection 2024 Oct 30. Heliyon. 2024. PMID: 39498056 Free PMC article. Review.
-
Neutrophil chemotaxis score and chemotaxis-related genes have the potential for clinical application to prognosticate the survival of patients with tumours.BMC Cancer. 2024 Oct 8;24(1):1244. doi: 10.1186/s12885-024-12993-1. BMC Cancer. 2024. PMID: 39379856 Free PMC article.
-
Advances in Immunomodulatory Mesoporous Silica Nanoparticles for Inflammatory and Cancer Therapies.Biomolecules. 2024 Aug 25;14(9):1057. doi: 10.3390/biom14091057. Biomolecules. 2024. PMID: 39334825 Free PMC article. Review.
-
Microenvironmental regulation of tumor-associated neutrophils in malignant glioma: from mechanism to therapy.J Neuroinflammation. 2024 Sep 16;21(1):226. doi: 10.1186/s12974-024-03222-4. J Neuroinflammation. 2024. PMID: 39285276 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

