Rab10 Phosphorylation is a Prominent Pathological Feature in Alzheimer's Disease
- PMID: 29562525
- PMCID: PMC6008156
- DOI: 10.3233/JAD-180023
Rab10 Phosphorylation is a Prominent Pathological Feature in Alzheimer's Disease
Abstract
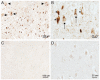
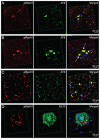
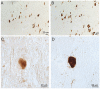
Alzheimer's disease (AD) is the leading cause of dementia in the elderly, characterized by neurofibrillary tangles (NFTs), senile plaques (SPs), and a progressive loss of neuronal cells in selective brain regions. Rab10, a small Rab GTPase involved in vesicular trafficking, has recently been identified as a novel protein associated with AD. Interestingly, Rab10 is a key substrate of leucine-rich repeat kinase 2 (LRRK2), a serine/threonine protein kinase genetically associated with the second most common neurodegenerative disease Parkinson's disease. However, the phosphorylation state of Rab10 has not yet been investigated in AD. Here, using a specific antibody recognizing LRRK2-mediated Rab10 phosphorylation at the amino acid residue threonine 73 (pRab10-T73), we performed immunocytochemical analysis of pRab10-T73 in hippocampal tissues of patients with AD. pRab10-T73 was prominent in NFTs in neurons within the hippocampus in all cases of AD examined, whereas immunoreactivity was very faint in control cases. Other characteristic AD pathological structures including granulovacuolar degeneration, dystrophic neurites and neuropil threads also contained pRab10-T73. The pRab10-T73 immunoreactivity was diminished greatly following dephosphorylation with alkaline phosphatase. pRab10-T73 was further found to be highly co-localized with hyperphosphorylated tau (pTau) in AD, and demonstrated similar pathological patterns as pTau in Down syndrome and progressive supranuclear palsy. Although pRab10-T73 immunoreactivity could be noted in dystrophic neurites surrounding SPs, SPs were largely negative for pRab10-T73. These findings indicate that Rab10 phosphorylation could be responsible for aberrations in the vesicle trafficking observed in AD leading to neurodegeneration.
Keywords: Alzheimer’s disease; dystrophic neurites; granulovacuolar degeneration; neurofibrillary tangles; neuropil threads; phosphorylated Rab10; senile plaques.
Figures

Similar articles
-
Cellular and subcellular localization of Rab10 and phospho-T73 Rab10 in the mouse and human brain.Acta Neuropathol Commun. 2023 Dec 18;11(1):201. doi: 10.1186/s40478-023-01704-9. Acta Neuropathol Commun. 2023. PMID: 38110990 Free PMC article.
-
Hypersialylation is a common feature of neurofibrillary tangles and granulovacuolar degenerations in Alzheimer's disease and tauopathy brains.Neuropathology. 2016 Aug;36(4):333-45. doi: 10.1111/neup.12277. Epub 2015 Dec 21. Neuropathology. 2016. PMID: 26685795
-
Current advances on different kinases involved in tau phosphorylation, and implications in Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2005 Jan;2(1):3-18. doi: 10.2174/1567205052772713. Curr Alzheimer Res. 2005. PMID: 15977985 Review.
-
LRRK2-mediated Rab10 phosphorylation in immune cells from Parkinson's disease patients.Mov Disord. 2019 Mar;34(3):406-415. doi: 10.1002/mds.27601. Epub 2018 Dec 30. Mov Disord. 2019. PMID: 30597610
-
Neuropathological diagnosis of Alzheimer's disease: a perspective from longitudinal clinicopathological studies.Neurobiol Aging. 1997 Jul-Aug;18(4 Suppl):S21-6. doi: 10.1016/s0197-4580(97)00065-1. Neurobiol Aging. 1997. PMID: 9330981 Review.
Cited by
-
A novel age-informed approach for genetic association analysis in Alzheimer's disease.Alzheimers Res Ther. 2021 Apr 1;13(1):72. doi: 10.1186/s13195-021-00808-5. Alzheimers Res Ther. 2021. PMID: 33794991 Free PMC article.
-
Therapeutic Targeting of Rab GTPases: Relevance for Alzheimer's Disease.Biomedicines. 2022 May 16;10(5):1141. doi: 10.3390/biomedicines10051141. Biomedicines. 2022. PMID: 35625878 Free PMC article. Review.
-
The Ras Superfamily of Small GTPases in Non-neoplastic Cerebral Diseases.Front Mol Neurosci. 2019 May 21;12:121. doi: 10.3389/fnmol.2019.00121. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31213978 Free PMC article. Review.
-
Impairment of the Glial Phagolysosomal System Drives Prion-Like Propagation in a Drosophila Model of Huntington's Disease.J Neurosci. 2024 May 15;44(20):e1256232024. doi: 10.1523/JNEUROSCI.1256-23.2024. J Neurosci. 2024. PMID: 38589228 Free PMC article.
-
Axonal transport of autophagosomes is regulated by dynein activators JIP3/JIP4 and ARF/RAB GTPases.bioRxiv [Preprint]. 2023 Jan 29:2023.01.28.526044. doi: 10.1101/2023.01.28.526044. bioRxiv. 2023. Update in: J Cell Biol. 2023 Dec 4;222(12):e202301084. doi: 10.1083/jcb.202301084. PMID: 36747648 Free PMC article. Updated. Preprint.
References
-
- Alloul K, Sauriol L, Kennedy W, Laurier C, Tessier G, Novosel S, Contandriopoulos A. Alzheimer’s disease: A review of the disease, its epidemiology and economic impact. Arch Gerontol Geriatr. 1998;27:189–221. - PubMed
-
- Smith MA. Alzheimer disease. Int Rev Neurobiol. 1998;42:1–54. - PubMed
-
- Czech C, Tremp G, Pradier L. Presenilins and Alzheimer’s disease: Biological functions and pathogenic mechanisms. Prog Neurobiol. 2000;60:363–384. - PubMed
-
- Fraser PE, Yang DS, Yu G, Levesque L, Nishimura M, Arawaka S, Serpell LC, Rogaeva E, St George-Hyslop P. Presenilin structure, function and role in Alzheimer disease. Biochim Biophys Acta. 2000;1502:1–15. - PubMed
-
- Tanahashi H, Tabira T. Alzheimer’s disease-associated presenilin 2 interacts with DRAL, an LIM-domain protein. Hum Mol Genet. 2000;9:2281–2289. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

