Conflict in the Intracellular Lives of Endosymbionts and Viruses: A Mechanistic Look at Wolbachia-Mediated Pathogen-blocking
- PMID: 29561780
- PMCID: PMC5923435
- DOI: 10.3390/v10040141
Conflict in the Intracellular Lives of Endosymbionts and Viruses: A Mechanistic Look at Wolbachia-Mediated Pathogen-blocking
Abstract
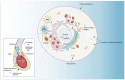
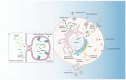
At the forefront of vector control efforts are strategies that leverage host-microbe associations to reduce vectorial capacity. The most promising of these efforts employs Wolbachia, a maternally transmitted endosymbiotic bacterium naturally found in 40% of insects. Wolbachia can spread through a population of insects while simultaneously inhibiting the replication of viruses within its host. Despite successes in using Wolbachia-transfected mosquitoes to limit dengue, Zika, and chikungunya transmission, the mechanisms behind pathogen-blocking have not been fully characterized. Firstly, we discuss how Wolbachia and viruses both require specific host-derived structures, compounds, and processes to initiate and maintain infection. There is significant overlap in these requirements, and infection with either microbe often manifests as cellular stress, which may be a key component of Wolbachia's anti-viral effect. Secondly, we discuss the current understanding of pathogen-blocking through this lens of cellular stress and develop a comprehensive view of how the lives of Wolbachia and viruses are fundamentally in conflict with each other. A thorough understanding of the genetic and cellular determinants of pathogen-blocking will significantly enhance the ability of vector control programs to deploy and maintain effective Wolbachia-mediated control measures.
Keywords: Aedes; Drosophila; antiviral; arbovirus; endosymbiont; symbiosis; vector control.
Conflict of interest statement
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures

Similar articles
-
Wolbachia and Virus Alter the Host Transcriptome at the Interface of Nucleotide Metabolism Pathways.mBio. 2021 Feb 9;12(1):e03472-20. doi: 10.1128/mBio.03472-20. mBio. 2021. PMID: 33563832 Free PMC article.
-
The RNAi pathway plays a small part in Wolbachia-mediated blocking of dengue virus in mosquito cells.Sci Rep. 2017 Mar 6;7:43847. doi: 10.1038/srep43847. Sci Rep. 2017. PMID: 28262718 Free PMC article.
-
Wolbachia-Conferred Antiviral Protection Is Determined by Developmental Temperature.mBio. 2021 Oct 26;12(5):e0292320. doi: 10.1128/mBio.02923-20. Epub 2021 Sep 7. mBio. 2021. PMID: 34488458 Free PMC article.
-
The Impact of Wolbachia on Virus Infection in Mosquitoes.Viruses. 2015 Nov 4;7(11):5705-17. doi: 10.3390/v7112903. Viruses. 2015. PMID: 26556361 Free PMC article. Review.
-
The Antiviral Effects of the Symbiont Bacteria Wolbachia in Insects.Front Immunol. 2021 Jan 29;11:626329. doi: 10.3389/fimmu.2020.626329. eCollection 2020. Front Immunol. 2021. PMID: 33584729 Free PMC article. Review.
Cited by
-
A tangled threesome: understanding arbovirus infection in Aedes spp. and the effect of the mosquito microbiota.Front Microbiol. 2024 Jan 3;14:1287519. doi: 10.3389/fmicb.2023.1287519. eCollection 2023. Front Microbiol. 2024. PMID: 38235434 Free PMC article. Review.
-
Pathogen blocking in Wolbachia-infected Aedes aegypti is not affected by Zika and dengue virus co-infection.PLoS Negl Trop Dis. 2019 May 20;13(5):e0007443. doi: 10.1371/journal.pntd.0007443. eCollection 2019 May. PLoS Negl Trop Dis. 2019. PMID: 31107912 Free PMC article.
-
Viral RNA is a target for Wolbachia-mediated pathogen blocking.PLoS Pathog. 2020 Jun 18;16(6):e1008513. doi: 10.1371/journal.ppat.1008513. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32555677 Free PMC article.
-
The Involvement of Atlastin in Dengue Virus and Wolbachia Infection in Aedes aegypti and Its Regulation by aae-miR-989.Microbiol Spectr. 2022 Oct 26;10(5):e0225822. doi: 10.1128/spectrum.02258-22. Epub 2022 Sep 27. Microbiol Spectr. 2022. PMID: 36165808 Free PMC article.
-
Assessing Aedes aegypti candidate genes during viral infection and Wolbachia-mediated pathogen blocking.Insect Mol Biol. 2022 Jun;31(3):356-368. doi: 10.1111/imb.12764. Epub 2022 Feb 14. Insect Mol Biol. 2022. PMID: 35112745 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

