Impact of RUNX2 on drug-resistant human pancreatic cancer cells with p53 mutations
- PMID: 29558908
- PMCID: PMC5861661
- DOI: 10.1186/s12885-018-4217-9
Impact of RUNX2 on drug-resistant human pancreatic cancer cells with p53 mutations
Abstract
Background: Despite the remarkable advances in the early diagnosis and treatment, overall 5-year survival rate of patients with pancreatic cancer is less than 10%. Gemcitabine (GEM), a cytidine nucleoside analogue and ribonucleotide reductase inhibitor, is a primary option for patients with advanced pancreatic cancer; however, its clinical efficacy is extremely limited. This unfavorable clinical outcome of pancreatic cancer patients is at least in part attributable to their poor response to anti-cancer drugs such as GEM. Thus, it is urgent to understand the precise molecular basis behind the drug-resistant property of pancreatic cancer and also to develop a novel strategy to overcome this deadly disease.
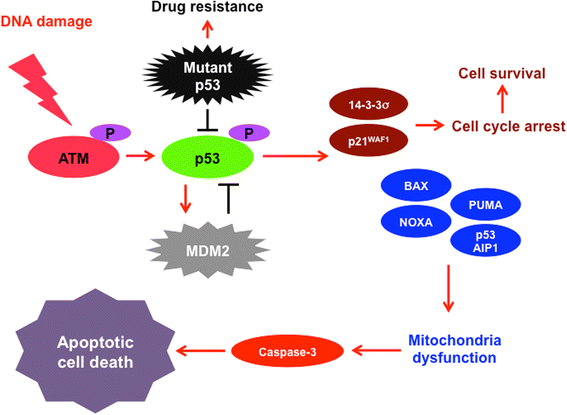
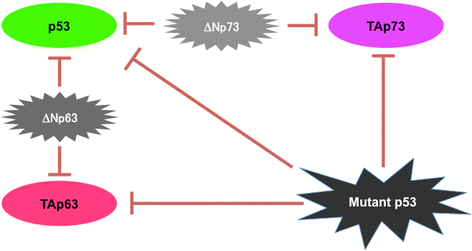
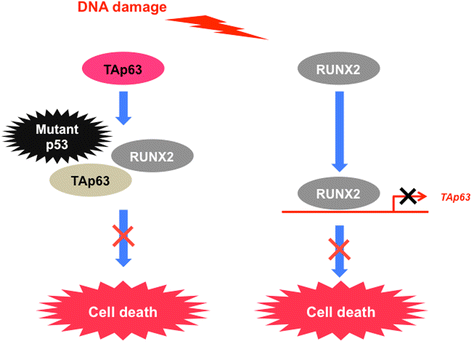
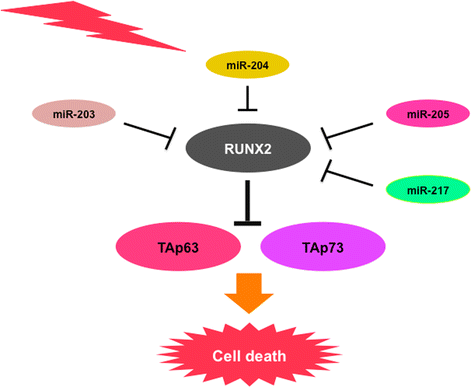
Review: Accumulating evidence strongly suggests that p53 mutations contribute to the acquisition and/or maintenance of drug-resistant property of pancreatic cancer. Indeed, certain p53 mutants render pancreatic cancer cells much more resistant to GEM, implying that p53 mutation is one of the critical determinants of GEM sensitivity. Intriguingly, runt-related transcription factor 2 (RUNX2) is expressed at higher level in numerous human cancers such as pancreatic cancer and osteosarcoma, indicating that, in addition to its pro-osteogenic role, RUNX2 has a pro-oncogenic potential. Moreover, a growing body of evidence implies that a variety of miRNAs suppress malignant phenotypes of pancreatic cancer cells including drug resistance through the down-regulation of RUNX2. Recently, we have found for the first time that forced depletion of RUNX2 significantly increases GEM sensitivity of p53-null as well as p53-mutated pancreatic cancer cells through the stimulation of p53 family TAp63/TAp73-dependent cell death pathway.
Conclusions: Together, it is likely that RUNX2 is one of the promising molecular targets for the treatment of the patients with pancreatic cancer regardless of their p53 status. In this review article, we will discuss how to overcome the serious drug-resistant phenotype of pancreatic cancer.
Keywords: Gemcitabine; Mutant p53; RUNX2; p53 family.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Depletion of pro-oncogenic RUNX2 enhances gemcitabine (GEM) sensitivity of p53-mutated pancreatic cancer Panc-1 cells through the induction of pro-apoptotic TAp63.Oncotarget. 2016 Nov 1;7(44):71937-71950. doi: 10.18632/oncotarget.12433. Oncotarget. 2016. PMID: 27713122 Free PMC article.
-
Impact of RUNX2 gene silencing on the gemcitabine sensitivity of p53‑mutated pancreatic cancer MiaPaCa‑2 spheres.Oncol Rep. 2018 Jun;39(6):2749-2758. doi: 10.3892/or.2018.6344. Epub 2018 Mar 30. Oncol Rep. 2018. PMID: 29620279
-
Silencing of RUNX2 enhances gemcitabine sensitivity of p53-deficient human pancreatic cancer AsPC-1 cells through the stimulation of TAp63-mediated cell death.Cell Death Dis. 2015 Oct 15;6(10):e1914. doi: 10.1038/cddis.2015.242. Cell Death Dis. 2015. PMID: 26469963 Free PMC article. No abstract available.
-
Novel Implications of DNA Damage Response in Drug Resistance of Malignant Cancers Obtained from the Functional Interaction between p53 Family and RUNX2.Biomolecules. 2015 Oct 23;5(4):2854-76. doi: 10.3390/biom5042854. Biomolecules. 2015. PMID: 26512706 Free PMC article. Review.
-
Targeting P53 as a Future Strategy to Overcome Gemcitabine Resistance in Biliary Tract Cancers.Biomolecules. 2020 Oct 23;10(11):1474. doi: 10.3390/biom10111474. Biomolecules. 2020. PMID: 33113997 Free PMC article. Review.
Cited by
-
Effects of Anti-Cancer Drug Sensitivity-Related Genetic Differences on Therapeutic Approaches in Refractory Papillary Thyroid Cancer.Int J Mol Sci. 2022 Jan 9;23(2):699. doi: 10.3390/ijms23020699. Int J Mol Sci. 2022. PMID: 35054884 Free PMC article.
-
Anti-Cancer SERCA Inhibitors Targeting Sorafenib-Resistant Human Papillary Thyroid Carcinoma.Int J Mol Sci. 2023 Apr 11;24(8):7069. doi: 10.3390/ijms24087069. Int J Mol Sci. 2023. PMID: 37108231 Free PMC article.
-
Non-syndromic supernumerary teeth and association with a self-reported family history of cancer.J Orofac Orthop. 2023 Dec 5. doi: 10.1007/s00056-023-00504-z. Online ahead of print. J Orofac Orthop. 2023. PMID: 38051344 English.
-
Enhanced osteopontin splicing regulated by RUNX2 is HDAC-dependent and induces invasive phenotypes in NSCLC cells.Cancer Cell Int. 2019 Nov 21;19:306. doi: 10.1186/s12935-019-1033-5. eCollection 2019. Cancer Cell Int. 2019. PMID: 31832019 Free PMC article.
-
Relevance of gene mutations and methylation to the growth of pancreatic intraductal papillary mucinous neoplasms based on pyrosequencing.Sci Rep. 2022 Jan 10;12(1):419. doi: 10.1038/s41598-021-04335-z. Sci Rep. 2022. PMID: 35013462 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

