Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells
- PMID: 29556359
- PMCID: PMC5858503
- DOI: 10.7150/thno.22172
Metal-based NanoEnhancers for Future Radiotherapy: Radiosensitizing and Synergistic Effects on Tumor Cells
Abstract
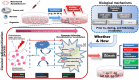
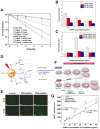
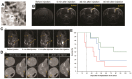
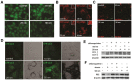
Radiotherapy is one of the major therapeutic strategies for cancer treatment. In the past decade, there has been growing interest in using high Z (atomic number) elements (materials) as radiosensitizers. New strategies in nanomedicine could help to improve cancer diagnosis and therapy at cellular and molecular levels. Metal-based nanoparticles usually exhibit chemical inertness in cellular and subcellular systems and may play a role in radiosensitization and synergistic cell-killing effects for radiation therapy. This review summarizes the efficacy of metal-based NanoEnhancers against cancers in both in vitro and in vivo systems for a range of ionizing radiations including gamma-rays, X-rays, and charged particles. The potential of translating preclinical studies on metal-based nanoparticles-enhanced radiation therapy into clinical practice is also discussed using examples of several metal-based NanoEnhancers (such as CYT-6091, AGuIX, and NBTXR3). Also, a few general examples of theranostic multimetallic nanocomposites are presented, and the related biological mechanisms are discussed.
Keywords: NanoEnhancers; metal-based nanoparticles; radiation therapy; radiosensitization; synergistic chemo-radiotherapy; tumor.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



Similar articles
-
Gold Nanoparticles as Radiosensitizers in Cancer Radiotherapy.Int J Nanomedicine. 2020 Nov 24;15:9407-9430. doi: 10.2147/IJN.S272902. eCollection 2020. Int J Nanomedicine. 2020. PMID: 33262595 Free PMC article. Review.
-
Recent Advances in Metal-Based NanoEnhancers for Particle Therapy.Nanomaterials (Basel). 2023 Mar 10;13(6):1011. doi: 10.3390/nano13061011. Nanomaterials (Basel). 2023. PMID: 36985905 Free PMC article. Review.
-
MRI-guided clinical 6-MV radiosensitization of glioma using a unique gadolinium-based nanoparticles injection.Nanomedicine (Lond). 2016 Sep;11(18):2405-17. doi: 10.2217/nnm-2016-0203. Epub 2016 Aug 16. Nanomedicine (Lond). 2016. PMID: 27529506
-
Role of nano-sensitizers in radiation therapy of metastatic tumors.Cancer Treat Res Commun. 2021;26:100303. doi: 10.1016/j.ctarc.2021.100303. Epub 2021 Jan 5. Cancer Treat Res Commun. 2021. PMID: 33454575 Review.
-
Nanoparticles for radiooncology: Mission, vision, challenges.Biomaterials. 2017 Mar;120:155-184. doi: 10.1016/j.biomaterials.2016.12.010. Epub 2016 Dec 14. Biomaterials. 2017. PMID: 28063356 Review.
Cited by
-
Recent Progress in Nanomedicine for Melanoma Theranostics With Emphasis on Combination Therapy.Front Bioeng Biotechnol. 2021 Mar 11;9:661214. doi: 10.3389/fbioe.2021.661214. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33777924 Free PMC article. Review.
-
Targeted anti-tumor synergistic effects of Myc decoy oligodeoxynucleotides-loaded selenium nanostructure combined with chemoradiotherapy on LNCaP prostate cancer cells.Oncol Res. 2023 Nov 15;32(1):101-125. doi: 10.32604/or.2023.044741. eCollection 2023. Oncol Res. 2023. PMID: 38188680 Free PMC article.
-
Delivery of Nanoparticle-Based Radiosensitizers for Radiotherapy Applications.Int J Mol Sci. 2019 Dec 31;21(1):273. doi: 10.3390/ijms21010273. Int J Mol Sci. 2019. PMID: 31906108 Free PMC article. Review.
-
Nanotechnology-based strategies for gastric cancer imaging and treatment.RSC Adv. 2021 Nov 2;11(56):35392-35407. doi: 10.1039/d1ra01947c. eCollection 2021 Oct 28. RSC Adv. 2021. PMID: 35493171 Free PMC article. Review.
-
The Coppery Age: Copper (Cu)-Involved Nanotheranostics.Adv Sci (Weinh). 2020 Aug 16;7(21):2001549. doi: 10.1002/advs.202001549. eCollection 2020 Nov. Adv Sci (Weinh). 2020. PMID: 33173728 Free PMC article. Review.
References
-
- Baumann M, Krause M, Overgaard J, Debus J, Bentzen SM, Daartz J. et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234–49. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

