Development of the Human Mycobiome over the First Month of Life and across Body Sites
- PMID: 29546248
- PMCID: PMC5840654
- DOI: 10.1128/mSystems.00140-17
Development of the Human Mycobiome over the First Month of Life and across Body Sites
Abstract
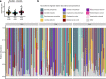
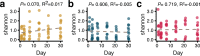
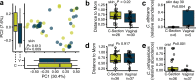
With the advent of next-generation sequencing and microbial community characterization, we are beginning to understand the key factors that shape early-life microbial colonization and associated health outcomes. Studies characterizing infant microbial colonization have focused mostly on bacteria in the microbiome and have largely neglected fungi (the mycobiome), despite their relevance to mucosal infections in healthy infants. In this pilot study, we characterized the skin, oral, and anal mycobiomes of infants over the first month of life (n = 17) and the anal and vaginal mycobiomes of mothers (n = 16) by internal transcribed spacer 2 (ITS2) amplicon sequencing. We found that infant mycobiomes differed by body site, with the infant mycobiomes at the anal sites being different from those at the skin and oral sites. The relative abundances of body site-specific taxa differed by birth mode, with significantly more Candida albicans fungi present on the skin of vaginally born infants on day 30 and significantly more Candida orthopsilosis fungi present in the oral cavity of caesarean section-born infants throughout the first month of life. We found the mycobiomes within individual infants to be variable over the first month of life, and vaginal birth did not result in infant mycobiomes that were more similar to the mother's vaginal mycobiome. Therefore, although vertical transmission of specific fungal isolates from mother to infant has been reported, it is likely that other sources (environment, other caregivers) also contribute to early-life mycobiome establishment. Thus, future longitudinal studies of mycobiome and bacterial microbiome codevelopment, with dense sampling from birth to beyond the first month of life, are warranted. IMPORTANCE Humans are colonized by diverse fungi (mycobiome), which have received much less study to date than colonizing bacteria. We know very little about the succession of fungal colonization in early life and whether it may relate to long-term health. To better understand fungal colonization and its sources, we studied the skin, oral, and anal mycobiomes of healthy term infants and the vaginal and anal mycobiomes of their mothers. Generally, infants were colonized by few fungal taxa, and fungal alpha diversity did not increase over the first month of life. There was no clear community maturation over the first month of life, regardless of body site. Key body-site-specific taxa, but not overall fungal community structures, were impacted by birth mode. Thus, additional studies to characterize mycobiome acquisition and succession throughout early life are needed to form a foundation for research into the relationship between mycobiome development and human disease.
Keywords: ITS2; fungi; infant; microbiome; mycobiome.
Figures


Similar articles
-
Critically Appraising the Significance of the Oral Mycobiome.J Dent Res. 2021 Feb;100(2):133-140. doi: 10.1177/0022034520956975. Epub 2020 Sep 13. J Dent Res. 2021. PMID: 32924741 Free PMC article. Review.
-
Infant Mode of Delivery Shapes the Skin Mycobiome of Prepubescent Children.Microbiol Spectr. 2022 Oct 26;10(5):e0226722. doi: 10.1128/spectrum.02267-22. Epub 2022 Sep 8. Microbiol Spectr. 2022. PMID: 36073919 Free PMC article.
-
Breastmilk and NICU surfaces are potential sources of fungi for infant mycobiomes.Fungal Genet Biol. 2019 Jul;128:29-35. doi: 10.1016/j.fgb.2019.03.008. Epub 2019 Mar 21. Fungal Genet Biol. 2019. PMID: 30905830 Free PMC article.
-
Bacterial, fungal, and interkingdom microbiome features of exclusively breastfeeding dyads are associated with infant age, antibiotic exposure, and birth mode.Front Microbiol. 2022 Nov 17;13:1050574. doi: 10.3389/fmicb.2022.1050574. eCollection 2022. Front Microbiol. 2022. PMID: 36466688 Free PMC article.
-
Infant fungal communities: current knowledge and research opportunities.BMC Med. 2017 Feb 13;15(1):30. doi: 10.1186/s12916-017-0802-z. BMC Med. 2017. PMID: 28190400 Free PMC article. Review.
Cited by
-
The Mycobiome in Health and Disease: Emerging Concepts, Methodologies and Challenges.Mycopathologia. 2020 Apr;185(2):207-231. doi: 10.1007/s11046-019-00413-z. Epub 2020 Jan 1. Mycopathologia. 2020. PMID: 31894501 Free PMC article. Review.
-
Influence of delivery and feeding mode in oral fungi colonization - a systematic review.Microb Cell. 2020 Jan 7;7(2):36-45. doi: 10.15698/mic2020.02.706. Microb Cell. 2020. PMID: 32025512 Free PMC article. Review.
-
Divergent maturational patterns of the infant bacterial and fungal gut microbiome in the first year of life are associated with inter-kingdom community dynamics and infant nutrition.Microbiome. 2024 Feb 7;12(1):22. doi: 10.1186/s40168-023-01735-3. Microbiome. 2024. PMID: 38326891 Free PMC article.
-
Critically Appraising the Significance of the Oral Mycobiome.J Dent Res. 2021 Feb;100(2):133-140. doi: 10.1177/0022034520956975. Epub 2020 Sep 13. J Dent Res. 2021. PMID: 32924741 Free PMC article. Review.
-
Child type 1 diabetes associated with mother vaginal bacteriome and mycobiome.Med Microbiol Immunol. 2022 Aug;211(4):185-194. doi: 10.1007/s00430-022-00741-w. Epub 2022 Jun 14. Med Microbiol Immunol. 2022. PMID: 35701558 Free PMC article.
References
-
- Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ, Britton HM, Lefebvre DL, Subbarao P, Mandhane P, Becker A, McNagny KM, Sears MR, Kollmann T; CHILD Study, Mohn WW, Turvey SE, Finlay BB. 2015. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med 7:307ra152. doi:10.1126/scitranslmed.aab2271. - DOI - PubMed
-
- Strati F, Di Paola M, Stefanini I, Albanese D, Rizzetto L, Lionetti P, Calabrò A, Jousson O, Donati C, Cavalieri D, De Filippo C. 2016. Age and gender affect the composition of fungal population of the human gastrointestinal tract. Front Microbiol 7:1227. doi:10.3389/fmicb.2016.01227. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
