MHC class I in dopaminergic neurons suppresses relapse to reward seeking
- PMID: 29546241
- PMCID: PMC5851664
- DOI: 10.1126/sciadv.aap7388
MHC class I in dopaminergic neurons suppresses relapse to reward seeking
Abstract
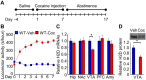
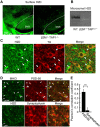
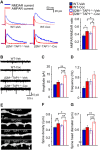
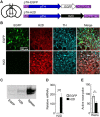
Major histocompatibility complex class I (MHCI) is an important immune protein that is expressed in various brain regions, with its deficiency leading to extensive synaptic transmission that results in learning and memory deficits. Although MHCI is highly expressed in dopaminergic neurons, its role in these neurons has not been examined. We show that MHCI expressed in dopaminergic neurons plays a key role in suppressing reward-seeking behavior. In wild-type mice, cocaine self-administration caused persistent reduction of MHCI specifically in dopaminergic neurons, which was accompanied by enhanced glutamatergic synaptic transmission and relapse to cocaine seeking. Functional MHCI knockout promoted this addictive phenotype for cocaine and a natural reward, namely, sucrose. In contrast, wild-type mice overexpressing a major MHCI gene (H2D) in dopaminergic neurons showed suppressed cocaine seeking. These results show that persistent cocaine-induced reduction of MHCI in dopaminergic neurons is necessary for relapse to cocaine seeking.
Figures

Similar articles
-
Locomotor- and Reward-Enhancing Effects of Cocaine Are Differentially Regulated by Chemogenetic Stimulation of Gi-Signaling in Dopaminergic Neurons.eNeuro. 2018 Jun 18;5(3):ENEURO.0345-17.2018. doi: 10.1523/ENEURO.0345-17.2018. eCollection 2018 May-Jun. eNeuro. 2018. PMID: 29938215 Free PMC article.
-
Knockdown of CRF1 receptors in the ventral tegmental area attenuates cue- and acute food deprivation stress-induced cocaine seeking in mice.J Neurosci. 2014 Aug 27;34(35):11560-70. doi: 10.1523/JNEUROSCI.4763-12.2014. J Neurosci. 2014. PMID: 25164654 Free PMC article.
-
Loss of the trpc4 gene is associated with a reduction in cocaine self-administration and reduced spontaneous ventral tegmental area dopamine neuronal activity, without deficits in learning for natural rewards.Behav Brain Res. 2016 Jun 1;306:117-27. doi: 10.1016/j.bbr.2016.03.027. Epub 2016 Mar 14. Behav Brain Res. 2016. PMID: 26988269
-
Chemogenetic Manipulations of Ventral Tegmental Area Dopamine Neurons Reveal Multifaceted Roles in Cocaine Abuse.J Neurosci. 2019 Jan 16;39(3):503-518. doi: 10.1523/JNEUROSCI.0537-18.2018. Epub 2018 Nov 16. J Neurosci. 2019. PMID: 30446532 Free PMC article.
-
Ventral tegmental glutamate: a role in stress-, cue-, and cocaine-induced reinstatement of cocaine-seeking.Neuropharmacology. 2009;56 Suppl 1(Suppl 1):174-6. doi: 10.1016/j.neuropharm.2008.06.008. Epub 2008 Jun 13. Neuropharmacology. 2009. PMID: 18598707 Free PMC article. Review.
Cited by
-
THE EXPOSOME IN HUMAN EVOLUTION: FROM DUST TO DIESEL.Q Rev Biol. 2019 Dec;94(4):333-394. doi: 10.1086/706768. Q Rev Biol. 2019. PMID: 32269391 Free PMC article.
-
A Sentinel in the Crosstalk Between the Nervous and Immune System: The (Immuno)-Proteasome.Front Immunol. 2019 Mar 29;10:628. doi: 10.3389/fimmu.2019.00628. eCollection 2019. Front Immunol. 2019. PMID: 30984192 Free PMC article. Review.
-
Risk assessment of substance use disorders based on the human leukocyte antigen (HLA).Sci Rep. 2023 May 26;13(1):8545. doi: 10.1038/s41598-023-35305-2. Sci Rep. 2023. PMID: 37237010 Free PMC article.
-
Conserved and cell type-specific transcriptional responses to IFN-γ in the ventral midbrain.Brain Behav Immun. 2023 Jul;111:277-291. doi: 10.1016/j.bbi.2023.04.008. Epub 2023 Apr 24. Brain Behav Immun. 2023. PMID: 37100211 Free PMC article.
-
Targeting Microglial Immunoproteasome: A Novel Approach in Neuroinflammatory-Related Disorders.ACS Chem Neurosci. 2024 Jul 17;15(14):2532-2544. doi: 10.1021/acschemneuro.4c00099. Epub 2024 Jul 6. ACS Chem Neurosci. 2024. PMID: 38970802 Free PMC article. Review.
References
-
- Ljunggren H. G., Van Kaer L., Sabatine M. S., Auchincloss H. Jr, Tonegawa S., Ploegh H. L., MHC class I expression and CD8+ T cell development in TAP1/beta 2-microglobulin double mutant mice. Int. Immunol. 7, 975–984 (1995). - PubMed
-
- Corriveau R. A., Huh G. S., Shatz C. J., Regulation of class I MHC gene expression in the developing and mature CNS by neural activity. Neuron 21, 505–520 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

