Lentivirus-mediated overexpression of OTULIN ameliorates microglia activation and neuroinflammation by depressing the activation of the NF-κB signaling pathway in cerebral ischemia/reperfusion rats
- PMID: 29544517
- PMCID: PMC5856386
- DOI: 10.1186/s12974-018-1117-5
Lentivirus-mediated overexpression of OTULIN ameliorates microglia activation and neuroinflammation by depressing the activation of the NF-κB signaling pathway in cerebral ischemia/reperfusion rats
Abstract
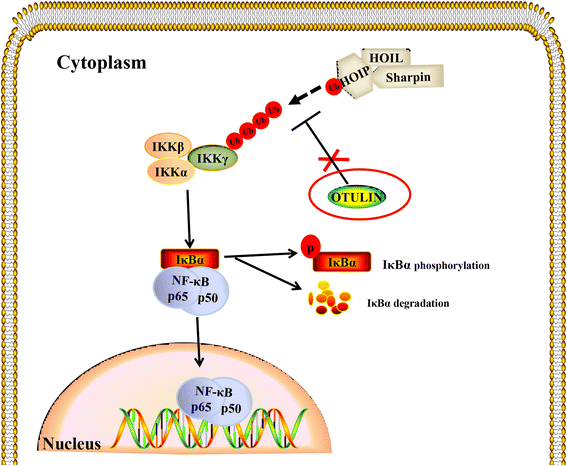
Background: Ischemic stroke-induced neuroinflammation is mainly mediated by microglial cells. The nuclear factor kappa B (NF-κB) pathway is the key transcriptional pathway that initiates inflammatory responses following cerebral ischemia. OTULIN, a critical negative regulator of the NF-κΒ signaling pathway, exerts robust effects on peripheral immune cell-mediated inflammation and is regarded as an essential mediator for repressing inflammation in vivo. The effect of OTULIN on inflammatory responses in the central nervous system (CNS) was previously unstudied. This current study investigated the anti-inflammatory effect of OTULIN both in vitro and in vivo in ischemic stroke models.
Methods: Sprague-Dawley (SD) rats were subjected to transient middle cerebral artery occlusion (tMCAO) or an intraperitoneal injection of lipopolysaccharide (LPS). Overexpression of the OTULIN gene was utilized to observe the effect of OTULIN on ischemic stroke outcomes. The effect of OTULIN overexpression on microglia-mediated neuroinflammation was examined in rat primary microglia (PM) and in the microglial cell line N9 after induction by oxygen-glucose deprivation (OGD)-treated neuronal medium. The activation and inflammatory responses of microglia were detected using immunofluorescence, ELISA, and qRT-PCR. The details of molecular mechanism were assessed using Western blotting.
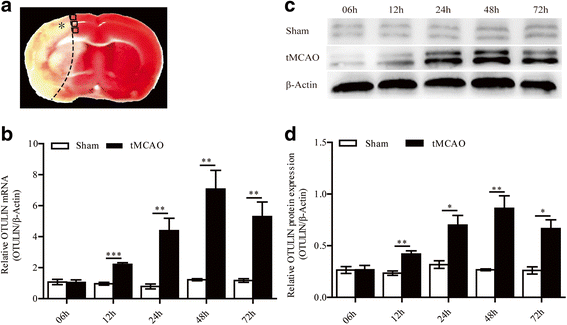
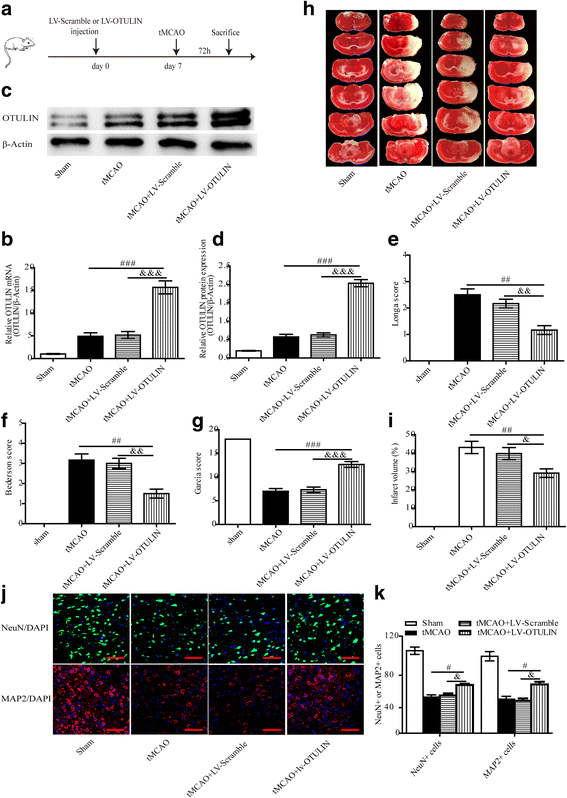
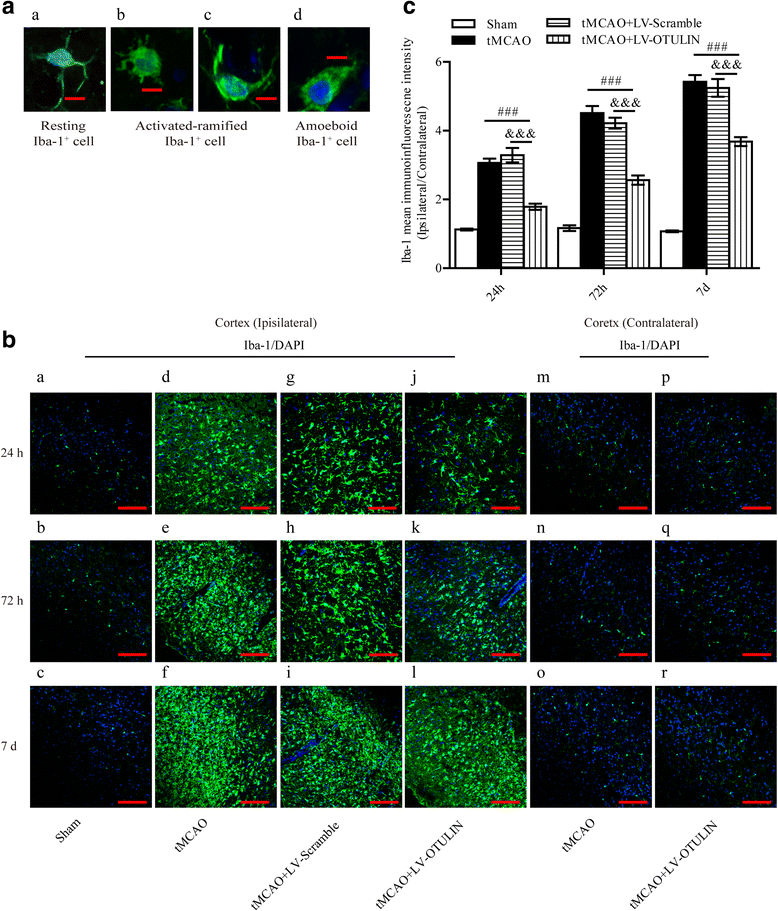
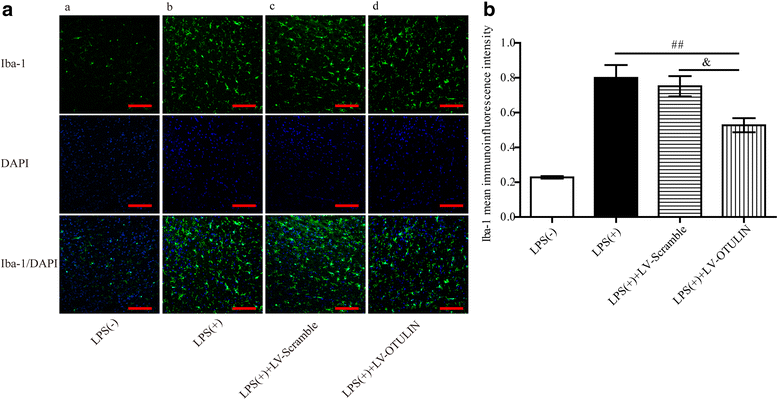
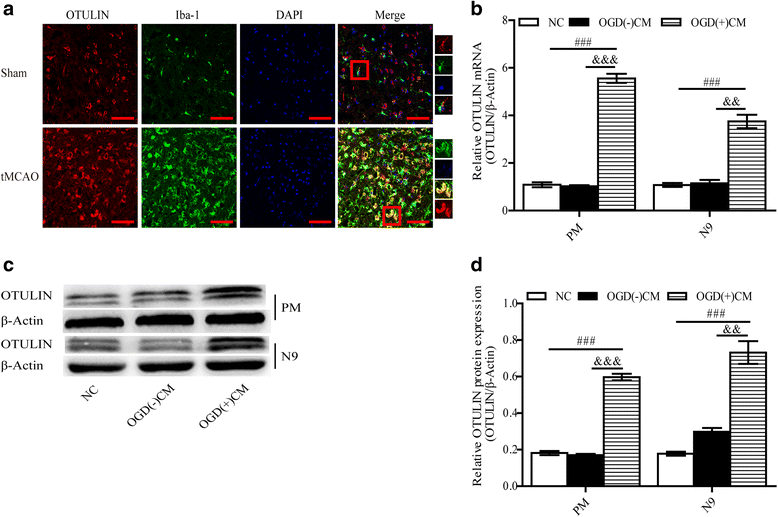
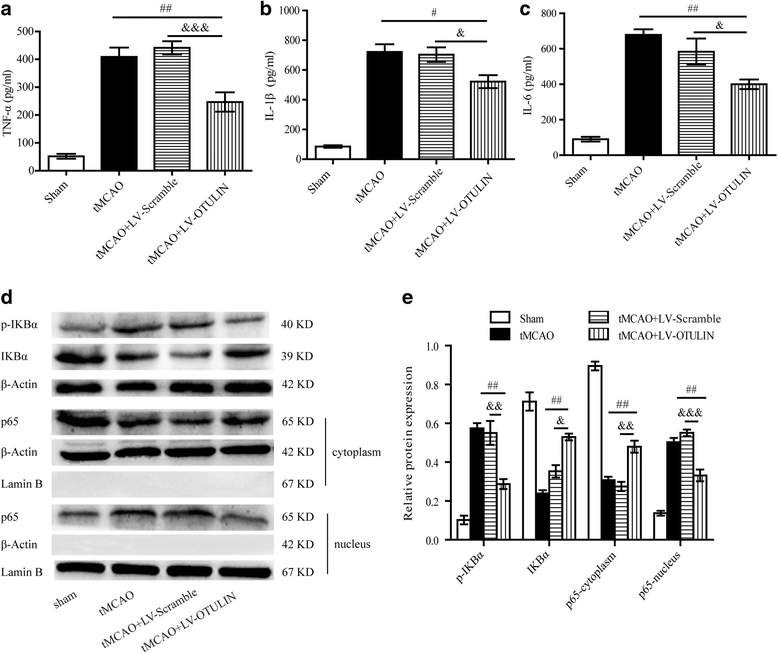
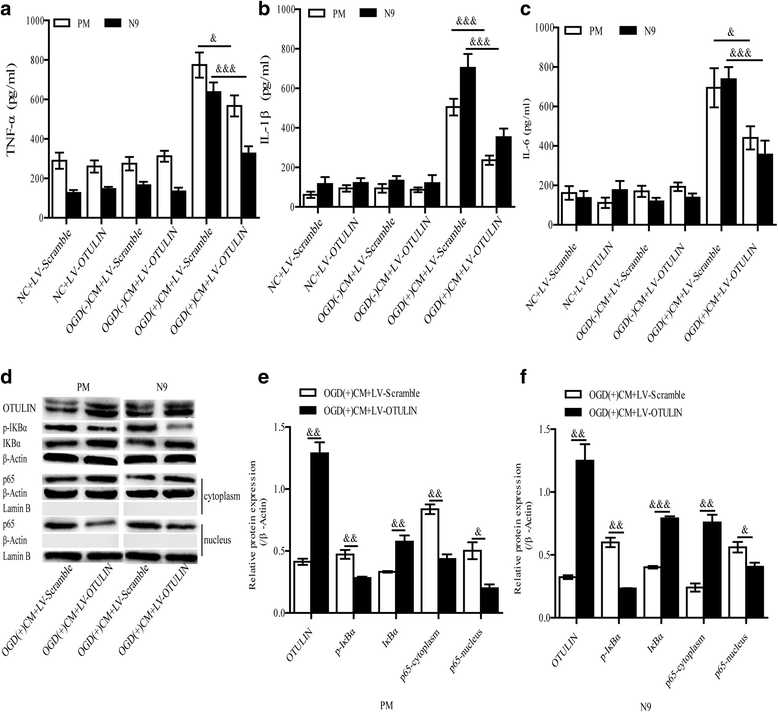
Results: In the tMCAO rats, the focal cerebral ischemia/reperfusion injury induced a continuous increase in OTULIN expression within 72 h, and OTULIN expression was increased in activated microglial cells. OTULIN overexpression obviously decreased the cerebral infarct volume, improved the neurological function deficits, and reduced neuronal loss at 72 h after reperfusion, and it also inhibited the activation of microglia and attenuated the release of TNF-α, IL-1β, and IL-6 by suppressing the NF-κB pathway at 24 h after tMCAO. In vitro, OTULIN overexpression inhibited the microglia-mediated neuroinflammation by reducing the production of TNF-α, IL-1β, and IL-6 via depressing the NF-κB pathway in both PM and N9 cells.
Conclusions: OTULIN provides a potential therapeutic target for ischemic brain injury by ameliorating the excessive activation of microglial cells and neuroinflammation through repressing the NF-κB signaling pathway.
Keywords: Cerebral ischemia/reperfusion; Microglia; NF-κB signaling pathway; Neuroinflammation; OTULIN.
Conflict of interest statement
Ethics approval
Our manuscript data were collected from animals and the study was approved by the Ethics Committee for Animal Experimentation of Chongqing Medical University. The reference number includes number of permit SYXK (渝) 2012–0001 and number of animal qualitative qualification 0001815.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
OTULIN is a new target of EA treatment in the alleviation of brain injury and glial cell activation via suppression of the NF-κB signalling pathway in acute ischaemic stroke rats.Mol Med. 2021 Apr 9;27(1):37. doi: 10.1186/s10020-021-00297-0. Mol Med. 2021. PMID: 33836646 Free PMC article.
-
Shikonin attenuates cerebral ischemia/reperfusion injury via inhibiting NOD2/RIP2/NF-κB-mediated microglia polarization and neuroinflammation.J Stroke Cerebrovasc Dis. 2024 Jun;33(6):107689. doi: 10.1016/j.jstrokecerebrovasdis.2024.107689. Epub 2024 Mar 26. J Stroke Cerebrovasc Dis. 2024. PMID: 38527567
-
Madecassoside protects BV2 microglial cells from oxygen-glucose deprivation/reperfusion-induced injury via inhibition of the toll-like receptor 4 signaling pathway.Brain Res. 2018 Jan 15;1679:144-154. doi: 10.1016/j.brainres.2017.11.030. Epub 2017 Dec 1. Brain Res. 2018. PMID: 29198964
-
Downregulation of Nogo-B ameliorates cerebral ischemia/reperfusion injury in mice through regulating microglia polarization via TLR4/NF-kappaB pathway.Neurochem Int. 2023 Jul;167:105553. doi: 10.1016/j.neuint.2023.105553. Epub 2023 May 23. Neurochem Int. 2023. PMID: 37230196 Review.
-
Loureirin B protects against cerebral ischemia/reperfusion injury through modulating M1/M2 microglial polarization via STAT6 / NF-kappaB signaling pathway.Eur J Pharmacol. 2023 Aug 15;953:175860. doi: 10.1016/j.ejphar.2023.175860. Epub 2023 Jun 16. Eur J Pharmacol. 2023. PMID: 37331681 Review.
Cited by
-
A20-Binding Inhibitor of NF-κB 1 Ameliorates Neuroinflammation and Mediates Antineuroinflammatory Effect of Electroacupuncture in Cerebral Ischemia/Reperfusion Rats.Evid Based Complement Alternat Med. 2020 Oct 13;2020:6980398. doi: 10.1155/2020/6980398. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 33110436 Free PMC article.
-
OTULIN's influence on neuroinflammation and pain modulation in trigeminal neuralgia.CNS Neurosci Ther. 2024 Aug;30(8):e70006. doi: 10.1111/cns.70006. CNS Neurosci Ther. 2024. PMID: 39169794 Free PMC article.
-
Integrative Analysis of Machine Learning and Molecule Docking Simulations for Ischemic Stroke Diagnosis and Therapy.Molecules. 2023 Nov 22;28(23):7704. doi: 10.3390/molecules28237704. Molecules. 2023. PMID: 38067435 Free PMC article.
-
LncRNA MIAT enhances cerebral ischaemia/reperfusion injury in rat model via interacting with EGLN2 and reduces its ubiquitin-mediated degradation.J Cell Mol Med. 2021 Nov;25(21):10140-10151. doi: 10.1111/jcmm.16950. Epub 2021 Oct 22. J Cell Mol Med. 2021. PMID: 34687132 Free PMC article.
-
Sertad1 Induces Neurological Injury after Ischemic Stroke via the CDK4/p-Rb Pathway.Mol Cells. 2022 Apr 30;45(4):216-230. doi: 10.14348/molcells.2021.0071. Mol Cells. 2022. PMID: 35014620 Free PMC article.
References
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–2223. doi: 10.1016/S0140-6736(12)61689-4. - DOI - PubMed
-
- Correction to: Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circ. 2017, 136:e196. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

