ATP Release Channels
- PMID: 29534490
- PMCID: PMC5877669
- DOI: 10.3390/ijms19030808
ATP Release Channels
Abstract
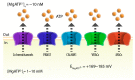
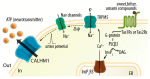
Adenosine triphosphate (ATP) has been well established as an important extracellular ligand of autocrine signaling, intercellular communication, and neurotransmission with numerous physiological and pathophysiological roles. In addition to the classical exocytosis, non-vesicular mechanisms of cellular ATP release have been demonstrated in many cell types. Although large and negatively charged ATP molecules cannot diffuse across the lipid bilayer of the plasma membrane, conductive ATP release from the cytosol into the extracellular space is possible through ATP-permeable channels. Such channels must possess two minimum qualifications for ATP permeation: anion permeability and a large ion-conducting pore. Currently, five groups of channels are acknowledged as ATP-release channels: connexin hemichannels, pannexin 1, calcium homeostasis modulator 1 (CALHM1), volume-regulated anion channels (VRACs, also known as volume-sensitive outwardly rectifying (VSOR) anion channels), and maxi-anion channels (MACs). Recently, major breakthroughs have been made in the field by molecular identification of CALHM1 as the action potential-dependent ATP-release channel in taste bud cells, LRRC8s as components of VRACs, and SLCO2A1 as a core subunit of MACs. Here, the function and physiological roles of these five groups of ATP-release channels are summarized, along with a discussion on the future implications of understanding these channels.
Keywords: ATP; CALHM; VRAC; VSOR; connexin; ion channel; maxi-anion channel; pannexin; purinergic signaling.
Conflict of interest statement
The author declares no conflict of interest.
Figures
Similar articles
-
Calcium homeostasis modulator (CALHM) ion channels.Pflugers Arch. 2016 Mar;468(3):395-403. doi: 10.1007/s00424-015-1757-6. Epub 2015 Nov 25. Pflugers Arch. 2016. PMID: 26603282 Free PMC article. Review.
-
Molecular Identities and ATP Release Activities of Two Types of Volume-Regulatory Anion Channels, VSOR and Maxi-Cl.Curr Top Membr. 2018;81:125-176. doi: 10.1016/bs.ctm.2018.07.004. Epub 2018 Aug 17. Curr Top Membr. 2018. PMID: 30243431 Review.
-
Maxi-anion channel and pannexin 1 hemichannel constitute separate pathways for swelling-induced ATP release in murine L929 fibrosarcoma cells.Am J Physiol Cell Physiol. 2012 Nov 1;303(9):C924-35. doi: 10.1152/ajpcell.00459.2011. Epub 2012 Jul 11. Am J Physiol Cell Physiol. 2012. PMID: 22785119
-
Differentiating connexin hemichannels and pannexin channels in cellular ATP release.FEBS Lett. 2014 Apr 17;588(8):1379-88. doi: 10.1016/j.febslet.2014.02.004. Epub 2014 Feb 15. FEBS Lett. 2014. PMID: 24548565 Free PMC article. Review.
-
Roles of volume-regulatory anion channels, VSOR and Maxi-Cl, in apoptosis, cisplatin resistance, necrosis, ischemic cell death, stroke and myocardial infarction.Curr Top Membr. 2019;83:205-283. doi: 10.1016/bs.ctm.2019.03.001. Epub 2019 Apr 19. Curr Top Membr. 2019. PMID: 31196606 Review.
Cited by
-
Activation of immune signals during organ transplantation.Signal Transduct Target Ther. 2023 Mar 11;8(1):110. doi: 10.1038/s41392-023-01377-9. Signal Transduct Target Ther. 2023. PMID: 36906586 Free PMC article. Review.
-
The Multifaceted Actions of CD73 During Development and Suppressive Actions of Regulatory T Cells.Front Immunol. 2022 May 31;13:914799. doi: 10.3389/fimmu.2022.914799. eCollection 2022. Front Immunol. 2022. PMID: 35711418 Free PMC article. Review.
-
Taste transduction and channel synapses in taste buds.Pflugers Arch. 2021 Jan;473(1):3-13. doi: 10.1007/s00424-020-02464-4. Epub 2020 Sep 16. Pflugers Arch. 2021. PMID: 32936320 Free PMC article. Review.
-
Cryo-EM structure of the heptameric calcium homeostasis modulator 1 channel.J Biol Chem. 2022 May;298(5):101838. doi: 10.1016/j.jbc.2022.101838. Epub 2022 Mar 24. J Biol Chem. 2022. PMID: 35339491 Free PMC article.
-
Probenecid Reduces Alcohol Drinking in Rodents. Is Pannexin1 a Novel Therapeutic Target for Alcohol Use Disorder?Alcohol Alcohol. 2019 Jan 9;54(5):497-502. doi: 10.1093/alcalc/agz054. Alcohol Alcohol. 2019. PMID: 31535696 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

