Integrin Activation: Implications for Axon Regeneration
- PMID: 29534450
- PMCID: PMC5870352
- DOI: 10.3390/cells7030020
Integrin Activation: Implications for Axon Regeneration
Abstract
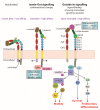
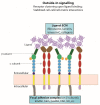
Integrin activation is essential for creating functional transmembrane receptors capable of inducing downstream cellular effects such as cell migration, cell spreading, neurite outgrowth and axon regeneration. Integrins are bidirectional signalling molecules that mediate their effects by 'inside-out' and 'outside-in' signalling. This review will provide a detailed overview of integrin activation focusing on intracellular activation in neurons and discussing direct implications in the regulation of neurite outgrowth and axon regeneration.
Keywords: extracellular matrix; gene therapy; integrin activation; kindlin; regeneration; talin; tenascin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Clutching at Guidance Cues: The Integrin-FAK Axis Steers Axon Outgrowth.Biology (Basel). 2023 Jul 3;12(7):954. doi: 10.3390/biology12070954. Biology (Basel). 2023. PMID: 37508384 Free PMC article. Review.
-
Integrin signalling and function in immune cells.Immunology. 2012 Apr;135(4):268-75. doi: 10.1111/j.1365-2567.2011.03549.x. Immunology. 2012. PMID: 22211918 Free PMC article. Review.
-
Integrin regulation.Curr Opin Cell Biol. 2005 Oct;17(5):509-16. doi: 10.1016/j.ceb.2005.08.010. Curr Opin Cell Biol. 2005. PMID: 16099636 Review.
-
Immunopathologies linked to integrin signalling.Semin Immunopathol. 2010 Jun;32(2):173-82. doi: 10.1007/s00281-010-0202-3. Epub 2010 Mar 10. Semin Immunopathol. 2010. PMID: 20217414 Review.
-
Talin-dependent integrin signalling in vivo.Thromb Haemost. 2009 Jun;101(6):1020-4. Thromb Haemost. 2009. PMID: 19492142 Review.
Cited by
-
Involvement of integrin αvβ3 in thyroid hormone-induced dendritogenesis.Front Endocrinol (Lausanne). 2022 Aug 22;13:938596. doi: 10.3389/fendo.2022.938596. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36072926 Free PMC article.
-
Induction of Neurogenesis and Angiogenesis in a Rat Hemisection Spinal Cord Injury Model With Combined Neural Stem Cell, Endothelial Progenitor Cell, and Biomimetic Hydrogel Matrix Therapy.Crit Care Explor. 2021 Jun 14;3(6):e0436. doi: 10.1097/CCE.0000000000000436. eCollection 2021 Jun. Crit Care Explor. 2021. PMID: 34151277 Free PMC article.
-
Recombinant Integrin β1 Signal Peptide Blocks Gliosis Induced by Aβ Oligomers.Int J Mol Sci. 2022 May 20;23(10):5747. doi: 10.3390/ijms23105747. Int J Mol Sci. 2022. PMID: 35628557 Free PMC article.
-
The Mechanotransduction Signaling Pathways in the Regulation of Osteogenesis.Int J Mol Sci. 2023 Sep 20;24(18):14326. doi: 10.3390/ijms241814326. Int J Mol Sci. 2023. PMID: 37762629 Free PMC article. Review.
-
Talin and Kindlin as Integrin-Activating Proteins: Focus on the Heart.Pediatr Cardiol. 2019 Oct;40(7):1401-1409. doi: 10.1007/s00246-019-02167-3. Epub 2019 Jul 31. Pediatr Cardiol. 2019. PMID: 31367953 Free PMC article. Review.
References
-
- Milner R., Huang X., Wu J., Nishimura S., Pytela R., Sheppard D., ffrench-Constant C. Distinct roles for astrocyte alphavbeta5 and alphavbeta8 integrins in adhesion and migration. Pt 23J. Cell Sci. 1999;112:4271–4279. - PubMed
-
- Feltri M.L., Graus Porta D., Previtali S.C., Nodari A., Migliavacca B., Cassetti A., Littlewood-Evans A., Reichardt L.F., Messing A., Quattrini A., et al. Conditional disruption of β 1 integrin in schwann cells impedes interactions with axons. J. Cell Biol. 2002;156:199–209. doi: 10.1083/jcb.200109021. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

