Heat shock protein-27 and sex-selective regulation of muscarinic and proteinase-activated receptor 2-mediated vasodilatation: differential sensitivity to endothelial NOS inhibition
- PMID: 29532457
- PMCID: PMC5979760
- DOI: 10.1111/bph.14200
Heat shock protein-27 and sex-selective regulation of muscarinic and proteinase-activated receptor 2-mediated vasodilatation: differential sensitivity to endothelial NOS inhibition
Abstract
Background and purpose: Previously, we demonstrated that exogenous heat shock protein 27 (HSP27/gene, HSPB1) treatment of human endothelial progenitor cells (EPCs) increases the synthesis and secretion of VEGF, improves EPC-migration/re-endothelialization and decreases neo-intima formation, suggesting a role for HSPB1 in regulating EPC function. We hypothesized that HSPB1 also affects mature endothelial cells (ECs) to alter EC-mediated vasoreactivity in vivo. Our work focused on endothelial NOS (eNOS)/NO-dependent relaxation induced by ACh and the coagulation pathway-activated receptor, proteinase-activated receptor 2 (PAR2).
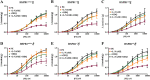
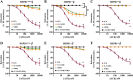
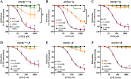
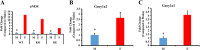
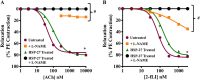
Experimental approach: Aorta rings from male and female wild-type, HSPB1-null and HSPB1 overexpressing (HSPB1o/e) mice were contracted with phenylephrine, and NOS-dependent relaxation responses to ACh and PAR2 agonist, 2-furoyl-LIGRLO-NH2 , were measured without and with L-NAME and ODQ, either alone or in combination to block NO synthesis/action. Tissues from female HSPB1-null mice were treated in vitro with recombinant HSP27 and then used for bioassay as above. Furthermore, oestrogen-specific effects were evaluated using a bioassay of aorta isolated from ovariectomized mice.
Key results: Relative to males, HSPB1-null female mice exhibited an increased L-NAME-resistant relaxation induced by activation of either PAR2 or muscarinic ACh receptors that was blocked in the concurrent presence of both L-NAME and ODQ. mRNAs (qPCR) for eNOS and ODQ-sensitive guanylyl-cyclase were increased in females versus males. Treatment of isolated aorta tissue with HSPB1 improved tissue responsiveness in the presence of L-NAME. Ovariectomy did not affect NO sensitivity, supporting an oestrogen-independent role for HSPB1.
Conclusions and implications: HSPB1 can regulate intact vascular endothelial function to affect NO-mediated vascular relaxation, especially in females.
© 2018 The British Pharmacological Society.
Figures

Similar articles
-
Perivascular adipose tissue-derived relaxing factors: release by peptide agonists via proteinase-activated receptor-2 (PAR2) and non-PAR2 mechanisms.Br J Pharmacol. 2011 Dec;164(8):1990-2002. doi: 10.1111/j.1476-5381.2011.01501.x. Br J Pharmacol. 2011. PMID: 21615723 Free PMC article.
-
Neuronal nitric oxide synthase-derived hydrogen peroxide is a major endothelium-dependent relaxing factor.Am J Physiol Heart Circ Physiol. 2008 Dec;295(6):H2503-11. doi: 10.1152/ajpheart.00731.2008. Epub 2008 Oct 24. Am J Physiol Heart Circ Physiol. 2008. PMID: 18952716
-
Effects induced by inhibitors of the phosphatidylinositol 3-kinase/Akt and nitric oxide synthase/guanylyl cyclase pathways on the isometric contraction in rat aorta: a comparative study.Fundam Clin Pharmacol. 2011 Jun;25(3):313-22. doi: 10.1111/j.1472-8206.2010.00833.x. Fundam Clin Pharmacol. 2011. PMID: 20584208
-
Protection of protease-activated receptor 2 mediated vasodilatation against angiotensin II-induced vascular dysfunction in mice.BMC Pharmacol. 2011 Sep 28;11:10. doi: 10.1186/1471-2210-11-10. BMC Pharmacol. 2011. PMID: 21955547 Free PMC article.
-
Multiple mechanisms of vascular smooth muscle relaxation by the activation of proteinase-activated receptor 2 in mouse mesenteric arterioles.Br J Pharmacol. 2002 Jan;135(1):155-69. doi: 10.1038/sj.bjp.0704469. Br J Pharmacol. 2002. PMID: 11786491 Free PMC article.
Cited by
-
Heat shock protein 27 activity is linked to endothelial barrier recovery after proinflammatory GPCR-induced disruption.Sci Signal. 2021 Aug 31;14(698):eabc1044. doi: 10.1126/scisignal.abc1044. Epub 2021 Aug 31. Sci Signal. 2021. PMID: 34516752 Free PMC article.
-
Single-cell RNA sequencing reveals cell type- and artery type-specific vascular remodelling in male spontaneously hypertensive rats.Cardiovasc Res. 2021 Mar 21;117(4):1202-1216. doi: 10.1093/cvr/cvaa164. Cardiovasc Res. 2021. PMID: 32589721 Free PMC article.
-
The pregnane X receptor and its microbiota-derived ligand indole 3-propionic acid regulate endothelium-dependent vasodilation.Am J Physiol Endocrinol Metab. 2019 Aug 1;317(2):E350-E361. doi: 10.1152/ajpendo.00572.2018. Epub 2019 Jun 18. Am J Physiol Endocrinol Metab. 2019. PMID: 31211619 Free PMC article.
-
Hsp20 Promotes Endothelial Progenitor Cell Angiogenesis via Activation of PI3K/Akt Signaling Pathway under Hypoxia.Tissue Eng Regen Med. 2022 Dec;19(6):1251-1266. doi: 10.1007/s13770-022-00481-1. Epub 2022 Aug 30. Tissue Eng Regen Med. 2022. PMID: 36042130 Free PMC article.
-
Validation of Housekeeping Genes as Reference for Reverse-Transcription-qPCR Analysis in Busulfan-Injured Microvascular Endothelial Cells.Biomed Res Int. 2018 Oct 2;2018:4953806. doi: 10.1155/2018/4953806. eCollection 2018. Biomed Res Int. 2018. PMID: 30386793 Free PMC article.
References
-
- Acunzo J, Katsogiannou M, Rocchi P (2012). Small heat shock proteins HSP27 (HspB1), alphaB‐crystallin (HspB5) and HSP22 (HspB8) as regulators of cell death. Int J Biochem Cell Biol 44: 1622–1631. - PubMed
-
- Al‐Ani B, Saifeddine M, Hollenberg MD (1995). Detection of functional receptors for the proteinase‐activated‐receptor‐2‐activating polypeptide, SLIGRL‐NH2, in rat vascular and gastric smooth muscle. Can J Physiol Pharmacol 73: 1203–1207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

