miR-296-3p Negatively Regulated by Nicotine Stimulates Cytoplasmic Translocation of c-Myc via MK2 to Suppress Chemotherapy Resistance
- PMID: 29525743
- PMCID: PMC6079479
- DOI: 10.1016/j.ymthe.2018.01.023
miR-296-3p Negatively Regulated by Nicotine Stimulates Cytoplasmic Translocation of c-Myc via MK2 to Suppress Chemotherapy Resistance
Abstract
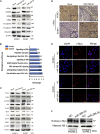
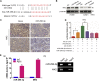
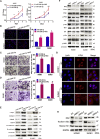
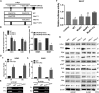
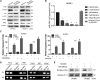
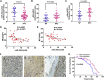
This study aimed to identify mechanisms by which microRNA 296-3p (miR-296-3p) functions as a tumor suppressor to restrain nasopharyngeal carcinoma (NPC) cell growth, metastasis, and chemoresistance. Mechanistic studies revealed that miR-296-3p negatively regulated by nicotine directly targets the oncogenic protein mitogen-activated protein kinase-activated protein kinase-2 (Mapkapk2) (MK2). Suppression of MK2 downregulated Ras/Braf/Erk/Mek/c-Myc and phosphoinositide-3-kinase (PI3K)/Akt/c-Myc signaling and promoted cytoplasmic translocation of c-Myc, which activated miR-296-3p expression by a feedback loop. This ultimately inhibited cell cycle progression, epithelial-to-mesenchymal transition (EMT), and chemoresistance of NPC. In addition, nicotine as a key component of tobacco was observed to suppress miR-296-3p and thus elevate MK2 expression by inducing PI3K/Akt/c-Myc signaling. In clinical samples, reduced miR-296-3p as an unfavorable factor was inversely correlated with MK2 and c-Myc expression. These results reveal a novel mechanism by which miR-296-3p negatively regulated by nicotine directly targets MK2-induced Ras/Braf/Erk/Mek/c-Myc or PI3K/AKT/c-Myc signaling to stimulate its own expression and suppress NPC cell proliferation and metastasis. miR-296-3p may thus serve as a therapeutic target to reverse chemotherapy resistance of NPC.
Keywords: MK2; miR-296-3p; nasopharyngeal carcinoma; nicotine.
Copyright © 2018 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
VPS33B interacts with NESG1 to modulate EGFR/PI3K/AKT/c-Myc/P53/miR-133a-3p signaling and induce 5-fluorouracil sensitivity in nasopharyngeal carcinoma.Cell Death Dis. 2019 Apr 3;10(4):305. doi: 10.1038/s41419-019-1457-9. Cell Death Dis. 2019. PMID: 30944308 Free PMC article.
-
miRomics and Proteomics Reveal a miR-296-3p/PRKCA/FAK/Ras/c-Myc Feedback Loop Modulated by HDGF/DDX5/β-catenin Complex in Lung Adenocarcinoma.Clin Cancer Res. 2017 Oct 15;23(20):6336-6350. doi: 10.1158/1078-0432.CCR-16-2813. Epub 2017 Jul 27. Clin Cancer Res. 2017. PMID: 28751441
-
A miR-567-PIK3AP1-PI3K/AKT-c-Myc feedback loop regulates tumour growth and chemoresistance in gastric cancer.EBioMedicine. 2019 Jun;44:311-321. doi: 10.1016/j.ebiom.2019.05.003. Epub 2019 May 9. EBioMedicine. 2019. PMID: 31078520 Free PMC article.
-
The outstanding role of miR-132-3p in carcinogenesis of solid tumors.Hum Cell. 2021 Jul;34(4):1051-1065. doi: 10.1007/s13577-021-00544-w. Epub 2021 May 17. Hum Cell. 2021. PMID: 33997944 Review.
-
Therapeutic targets of cancer drugs: Modulation by melatonin.Life Sci. 2021 Feb 15;267:118934. doi: 10.1016/j.lfs.2020.118934. Epub 2020 Dec 29. Life Sci. 2021. PMID: 33385405 Review.
Cited by
-
Long non-coding RNA MAGEA4-AS1 binding to p53 enhances MK2 signaling pathway and promotes the proliferation and metastasis of oral squamous cell carcinoma.Funct Integr Genomics. 2024 Sep 9;24(5):158. doi: 10.1007/s10142-024-01436-6. Funct Integr Genomics. 2024. PMID: 39249547 Free PMC article.
-
Nicotine Suppresses Phagocytic Ability of Macrophages by Regulating the miR-296-3p-SIRPα Axis.Anal Cell Pathol (Amst). 2023 Feb 15;2023:6306358. doi: 10.1155/2023/6306358. eCollection 2023. Anal Cell Pathol (Amst). 2023. PMID: 36845756 Free PMC article.
-
VPS33B interacts with NESG1 to modulate EGFR/PI3K/AKT/c-Myc/P53/miR-133a-3p signaling and induce 5-fluorouracil sensitivity in nasopharyngeal carcinoma.Cell Death Dis. 2019 Apr 3;10(4):305. doi: 10.1038/s41419-019-1457-9. Cell Death Dis. 2019. PMID: 30944308 Free PMC article.
-
miR-3682-3p directly targets FOXO3 and stimulates tumor stemness in hepatocellular carcinoma via a positive feedback loop involving FOXO3/PI3K/AKT/c-Myc.World J Stem Cells. 2022 Jul 26;14(7):539-555. doi: 10.4252/wjsc.v14.i7.539. World J Stem Cells. 2022. PMID: 36157524 Free PMC article.
-
Non-coding RNA and drug resistance in head and neck cancer.Cancer Drug Resist. 2024 Sep 20;7:34. doi: 10.20517/cdr.2024.59. eCollection 2024. Cancer Drug Resist. 2024. PMID: 39403599 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

