Increased macrophage-derived SPARC precedes collagen deposition in myocardial fibrosis
- PMID: 29522370
- PMCID: PMC6087775
- DOI: 10.1152/ajpheart.00719.2017
Increased macrophage-derived SPARC precedes collagen deposition in myocardial fibrosis
Abstract
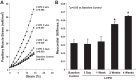
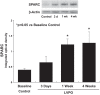
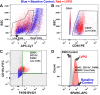
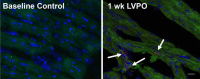
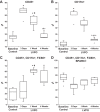
Myocardial fibrosis and the resultant increases in left ventricular stiffness represent pivotal consequences of chronic pressure overload (PO) that impact both functional capacity and the rates of morbid and mortal events. However, the time course and cellular mechanisms that underlie PO-induced fibrosis have not been completely defined. Secreted protein acidic and rich in cysteine (SPARC) is a matricellular protein that has been shown to be required for insoluble collagen deposition and increased myocardial stiffness in response to PO in mice. As macrophages are associated with increases in fibrillar collagen, the hypothesis that macrophages represent a source of increased SPARC production in the PO myocardium was tested. The time course of changes in the myocardial macrophage population was compared with changes in procollagen type I mRNA, production of SPARC, fibrillar collagen accumulation, and diastolic stiffness. In PO hearts, mRNA encoding collagen type I was increased at 3 days, whereas increases in levels of total collagen protein did not occur until 1 wk and were followed by increases in insoluble collagen at 2 wk. Increases in muscle stiffness were not detected before increases in insoluble collagen content (>1 wk). Significant increases in myocardial macrophages that coincided with increased SPARC were found but did not coincide with increases in mRNA encoding collagen type I. Furthermore, immunohistochemistry and flow cytometry identified macrophages as a cellular source of SPARC. We conclude that myocardial macrophages play an important role in the time-dependent increases in SPARC that enhance postsynthetic collagen processing, insoluble collagen content, and myocardial stiffness and contribute to the development of fibrosis. NEW & NOTEWORTHY Myocardial fibrosis and the resultant increases in left ventricular and myocardial stiffness represent pivotal consequences of chronic pressure overload. In this study a murine model of cardiac fibrosis induced by pressure overload was used to establish a time course of collagen expression, collagen deposition, and cardiac macrophage expansion.
Keywords: collagen; extracellular matrix; matricellular proteins; postsynthetic collagen processing; secreted protein acidic and rich in cysteine.
Figures
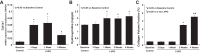

Similar articles
-
SPARC production by bone marrow-derived cells contributes to myocardial fibrosis in pressure overload.Am J Physiol Heart Circ Physiol. 2021 Feb 1;320(2):H604-H612. doi: 10.1152/ajpheart.00552.2020. Epub 2020 Dec 11. Am J Physiol Heart Circ Physiol. 2021. PMID: 33306449 Free PMC article.
-
Time course of right ventricular pressure-overload induced myocardial fibrosis: relationship to changes in fibroblast postsynthetic procollagen processing.Am J Physiol Heart Circ Physiol. 2012 Nov 1;303(9):H1128-34. doi: 10.1152/ajpheart.00482.2012. Epub 2012 Aug 31. Am J Physiol Heart Circ Physiol. 2012. PMID: 22942178 Free PMC article.
-
Pressure overload-induced alterations in fibrillar collagen content and myocardial diastolic function: role of secreted protein acidic and rich in cysteine (SPARC) in post-synthetic procollagen processing.Circulation. 2009 Jan 20;119(2):269-80. doi: 10.1161/CIRCULATIONAHA.108.773424. Epub 2008 Dec 31. Circulation. 2009. PMID: 19118257 Free PMC article.
-
The role of secreted protein acidic and rich in cysteine (SPARC) in cardiac repair and fibrosis: Does expression of SPARC by macrophages influence outcomes?J Mol Cell Cardiol. 2016 Apr;93:156-61. doi: 10.1016/j.yjmcc.2015.11.014. Epub 2015 Nov 12. J Mol Cell Cardiol. 2016. PMID: 26582465 Review.
-
Cardiac extracellular matrix remodeling: fibrillar collagens and Secreted Protein Acidic and Rich in Cysteine (SPARC).J Mol Cell Cardiol. 2010 Mar;48(3):544-9. doi: 10.1016/j.yjmcc.2009.06.018. Epub 2009 Jul 3. J Mol Cell Cardiol. 2010. PMID: 19577572 Free PMC article. Review.
Cited by
-
The Non-Fibrillar Side of Fibrosis: Contribution of the Basement Membrane, Proteoglycans, and Glycoproteins to Myocardial Fibrosis.J Cardiovasc Dev Dis. 2019 Sep 23;6(4):35. doi: 10.3390/jcdd6040035. J Cardiovasc Dev Dis. 2019. PMID: 31547598 Free PMC article. Review.
-
T-cell regulation of fibroblasts and cardiac fibrosis.Matrix Biol. 2020 Sep;91-92:167-175. doi: 10.1016/j.matbio.2020.04.001. Epub 2020 May 11. Matrix Biol. 2020. PMID: 32438054 Free PMC article. Review.
-
Reduced fibrillar collagen accumulation in skeletal muscle of secreted protein acidic and rich in cysteine (SPARC)-null mice.J Vet Med Sci. 2019 Nov 29;81(11):1649-1654. doi: 10.1292/jvms.19-0485. Epub 2019 Oct 2. J Vet Med Sci. 2019. PMID: 31582603 Free PMC article.
-
The Role of Matrix Proteins in Cardiac Pathology.Int J Mol Sci. 2022 Jan 25;23(3):1338. doi: 10.3390/ijms23031338. Int J Mol Sci. 2022. PMID: 35163259 Free PMC article. Review.
-
Left Ventricular Gene Expression in Heart Failure With Preserved Ejection Fraction-Profibrotic and Proinflammatory Pathways and Genes.Circ Heart Fail. 2023 Aug;16(8):e010395. doi: 10.1161/CIRCHEARTFAILURE.123.010395. Epub 2023 Jul 27. Circ Heart Fail. 2023. PMID: 37582166 Free PMC article.
References
-
- Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Müller AM, Volz KS, Tang Z, Red-Horse K, Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res 115: 625–635, 2014. doi:10.1161/CIRCRESAHA.115.303794. - DOI - PubMed
-
- Arnold SA, Rivera LB, Miller AF, Carbon JG, Dineen SP, Xie Y, Castrillon DH, Sage EH, Puolakkainen P, Bradshaw AD, Brekken RA. Lack of host SPARC enhances vascular function and tumor spread in an orthotopic murine model of pancreatic carcinoma. Dis Model Mech 3: 57–72, 2010. doi:10.1242/dmm.003228. - DOI - PMC - PubMed
-
- Baicu CF, Li J, Zhang Y, Kasiganesan H, Cooper G IV, Zile MR, Bradshaw AD. Time course of right ventricular pressure-overload induced myocardial fibrosis: relationship to changes in fibroblast postsynthetic procollagen processing. Am J Physiol Heart Circ Physiol 303: H1128–H1134, 2012. doi:10.1152/ajpheart.00482.2012. - DOI - PMC - PubMed
-
- Baicu CF, Zhang Y, Van Laer AO, Renaud L, Zile MR, Bradshaw AD. Effects of the absence of procollagen C-endopeptidase enhancer-2 on myocardial collagen accumulation in chronic pressure overload. Am J Physiol Heart Circ Physiol 303: H234–H240, 2012. doi:10.1152/ajpheart.00227.2012. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

