Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance
- PMID: 29515571
- PMCID: PMC5826312
- DOI: 10.3389/fimmu.2018.00230
Nanoparticles for the Induction of Antigen-Specific Immunological Tolerance
Abstract
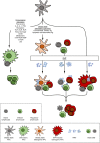
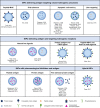
Antigen-specific immune tolerance has been a long-standing goal for immunotherapy for the treatment of autoimmune diseases and allergies and for the prevention of allograft rejection and anti-drug antibodies directed against biologic therapies. Nanoparticles have emerged as powerful tools to initiate and modulate immune responses due to their inherent capacity to target antigen-presenting cells (APCs) and deliver coordinated signals that can elicit an antigen-specific immune response. A wide range of strategies have been described to create tolerogenic nanoparticles (tNPs) that fall into three broad categories. One strategy includes tNPs that provide antigen alone to harness natural tolerogenic processes and environments, such as presentation of antigen in the absence of costimulatory signals, oral tolerance, the tolerogenic environment of the liver, and apoptotic cell death. A second strategy includes tNPs that carry antigen and simultaneously target tolerogenic receptors, such as pro-tolerogenic cytokine receptors, aryl hydrocarbon receptor, FAS receptor, and the CD22 inhibitory receptor. A third strategy includes tNPs that carry a payload of tolerogenic pharmacological agents that can "lock" APCs into a developmental or metabolic state that favors tolerogenic presentation of antigens. These diverse strategies have led to the development of tNPs that are capable of inducing antigen-specific immunological tolerance, not just immunosuppression, in animal models. These novel tNP technologies herald a promising approach to specifically prevent and treat unwanted immune reactions in humans. The first tNP, SEL-212, a biodegradable synthetic vaccine particle encapsulating rapamycin, has reached the clinic and is currently in Phase 2 clinical trials.
Keywords: immunological tolerance; nanoparticles; rapamycin; regulatory T cells; tolerogenic dendritic cells.
Figures
Similar articles
-
Polymeric synthetic nanoparticles for the induction of antigen-specific immunological tolerance.Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):E156-65. doi: 10.1073/pnas.1408686111. Epub 2014 Dec 29. Proc Natl Acad Sci U S A. 2015. PMID: 25548186 Free PMC article.
-
Induction of tolerogenic dendritic cells by vitamin D receptor agonists.Handb Exp Pharmacol. 2009;(188):251-73. doi: 10.1007/978-3-540-71029-5_12. Handb Exp Pharmacol. 2009. PMID: 19031030 Review.
-
Tolerogenic Nanoparticles Induce Antigen-Specific Regulatory T Cells and Provide Therapeutic Efficacy and Transferrable Tolerance against Experimental Autoimmune Encephalomyelitis.Front Immunol. 2018 Mar 2;9:281. doi: 10.3389/fimmu.2018.00281. eCollection 2018. Front Immunol. 2018. PMID: 29552007 Free PMC article.
-
Development of ImmTOR Tolerogenic Nanoparticles for the Mitigation of Anti-drug Antibodies.Front Immunol. 2020 May 20;11:969. doi: 10.3389/fimmu.2020.00969. eCollection 2020. Front Immunol. 2020. PMID: 32508839 Free PMC article. Review.
-
The impact of nanocarriers in the induction of antigen-specific immunotolerance in autoimmune diseases.J Control Release. 2021 Nov 10;339:274-283. doi: 10.1016/j.jconrel.2021.09.037. Epub 2021 Sep 29. J Control Release. 2021. PMID: 34600024 Review.
Cited by
-
Immunologically Inert Nanostructures as Selective Therapeutic Tools in Inflammatory Diseases.Cells. 2021 Mar 23;10(3):707. doi: 10.3390/cells10030707. Cells. 2021. PMID: 33806746 Free PMC article. Review.
-
Rusty Microglia: Trainers of Innate Immunity in Alzheimer's Disease.Front Neurol. 2018 Dec 4;9:1062. doi: 10.3389/fneur.2018.01062. eCollection 2018. Front Neurol. 2018. PMID: 30564191 Free PMC article. Review.
-
Intestinal Microbiota in Common Chronic Inflammatory Disorders Affecting Children.Front Immunol. 2021 Jun 7;12:642166. doi: 10.3389/fimmu.2021.642166. eCollection 2021. Front Immunol. 2021. PMID: 34163468 Free PMC article. Review.
-
How Advanced are Cancer Immuno-Nanotherapeutics? A Comprehensive Review of the Literature.Int J Nanomedicine. 2023 Jan 5;18:35-48. doi: 10.2147/IJN.S388349. eCollection 2023. Int J Nanomedicine. 2023. PMID: 36636642 Free PMC article. Review.
-
Targeting DCs for Tolerance Induction: Don't Lose Sight of the Neutrophils.Front Immunol. 2021 Oct 5;12:732992. doi: 10.3389/fimmu.2021.732992. eCollection 2021. Front Immunol. 2021. PMID: 34675923 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

