A20/Tumor Necrosis Factor α-Induced Protein 3 in Immune Cells Controls Development of Autoinflammation and Autoimmunity: Lessons from Mouse Models
- PMID: 29515565
- PMCID: PMC5826380
- DOI: 10.3389/fimmu.2018.00104
A20/Tumor Necrosis Factor α-Induced Protein 3 in Immune Cells Controls Development of Autoinflammation and Autoimmunity: Lessons from Mouse Models
Abstract
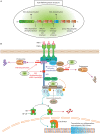
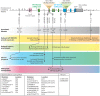
Immune cell activation is a stringently regulated process, as exaggerated innate and adaptive immune responses can lead to autoinflammatory and autoimmune diseases. Perhaps the best-characterized molecular pathway promoting cell activation is the nuclear factor-κB (NF-κB) signaling pathway. Stimulation of this pathway leads to transcription of numerous pro-inflammatory and cell-survival genes. Several mechanisms tightly control NF-κB activity, including the key regulatory zinc finger (de)ubiquitinating enzyme A20/tumor necrosis factor α-induced protein 3 (TNFAIP3). Single nucleotide polymorphisms (SNPs) in the vicinity of the TNFAIP3 gene are associated with a spectrum of chronic systemic inflammatory diseases, indicative of its clinical relevance. Mice harboring targeted cell-specific deletions of the Tnfaip3 gene in innate immune cells such as macrophages spontaneously develop autoinflammatory disease. When immune cells involved in the adaptive immune response, such as dendritic cells or B-cells, are targeted for A20/TNFAIP3 deletion, mice develop spontaneous inflammation that resembles human autoimmune disease. Therefore, more knowledge on A20/TNFAIP3 function in cells of the immune system is beneficial in our understanding of autoinflammation and autoimmunity. Using the aforementioned mouse models, novel A20/TNFAIP3 functions have recently been described including control of necroptosis and inflammasome activity. In this review, we discuss the function of the A20/TNFAIP3 enzyme and its critical role in various innate and adaptive immune cells. Finally, we discuss the latest findings on TNFAIP3 SNPs in human autoinflammatory and autoimmune diseases and address that genotyping of TNFAIP3 SNPs may guide treatment decisions.
Keywords: A20; NF-κB; autoimmune disease; autoinflammation; mouse models; single nucleotide polymorphisms; tumor necrosis factor α-induced protein 3; ubiquitination.
Figures
Similar articles
-
DNGR1-mediated deletion of A20/Tnfaip3 in dendritic cells alters T and B-cell homeostasis and promotes autoimmune liver pathology.J Autoimmun. 2019 Aug;102:167-178. doi: 10.1016/j.jaut.2019.05.007. Epub 2019 Jul 9. J Autoimmun. 2019. PMID: 31151831
-
Single nucleotide polymorphisms at the TNFAIP3/A20 locus and susceptibility/resistance to inflammatory and autoimmune diseases.Adv Exp Med Biol. 2014;809:163-83. doi: 10.1007/978-1-4939-0398-6_10. Adv Exp Med Biol. 2014. PMID: 25302371 Review.
-
B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice.Blood. 2011 Feb 17;117(7):2227-36. doi: 10.1182/blood-2010-09-306019. Epub 2010 Nov 18. Blood. 2011. PMID: 21088135
-
Expression, biological activities and mechanisms of action of A20 (TNFAIP3).Biochem Pharmacol. 2010 Dec 15;80(12):2009-20. doi: 10.1016/j.bcp.2010.06.044. Epub 2010 Jul 3. Biochem Pharmacol. 2010. PMID: 20599425 Review.
-
A20 Orchestrates Inflammatory Response in the Oral Mucosa through Restraining NF-κB Activity.J Immunol. 2019 Apr 1;202(7):2044-2056. doi: 10.4049/jimmunol.1801286. Epub 2019 Feb 13. J Immunol. 2019. PMID: 30760622 Free PMC article.
Cited by
-
Borrelia burgdorferi-Induced Changes in the Class II Self-Immunopeptidome Displayed on HLA-DR Molecules Expressed by Dendritic Cells.Front Med (Lausanne). 2020 Sep 16;7:568. doi: 10.3389/fmed.2020.00568. eCollection 2020. Front Med (Lausanne). 2020. PMID: 33043033 Free PMC article.
-
Overexpression of the ubiquitin-editing enzyme A20 in the brain lesions of Multiple Sclerosis patients: moving from systemic to central nervous system inflammation.Brain Pathol. 2021 Mar;31(2):283-296. doi: 10.1111/bpa.12906. Epub 2020 Nov 18. Brain Pathol. 2021. PMID: 33051914 Free PMC article.
-
A Case of Adult-Onset Still's Disease Caused by a Novel Splicing Mutation in TNFAIP3 Successfully Treated With Tocilizumab.Front Immunol. 2018 Jul 4;9:1527. doi: 10.3389/fimmu.2018.01527. eCollection 2018. Front Immunol. 2018. PMID: 30022980 Free PMC article.
-
Molecular biology of autoinflammatory diseases.Inflamm Regen. 2021 Oct 11;41(1):33. doi: 10.1186/s41232-021-00181-8. Inflamm Regen. 2021. PMID: 34635190 Free PMC article. Review.
-
Tumour Necrosis Factor Alpha-induced Protein 3 Negatively Regulates Cutibacterium acnes-induced Innate Immune Events in Epidermal Keratinocytes.Acta Derm Venereol. 2021 Jan 13;101(1):adv00369. doi: 10.2340/00015555-3707. Acta Derm Venereol. 2021. PMID: 33241420 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

