New insights into the formation and the function of lamellipodia and ruffles in mesenchymal cell migration
- PMID: 29513145
- PMCID: PMC6363039
- DOI: 10.1080/19336918.2018.1448352
New insights into the formation and the function of lamellipodia and ruffles in mesenchymal cell migration
Abstract
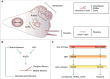
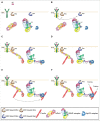
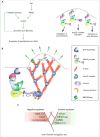
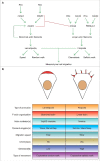
Lamellipodia and ruffles are veil-shaped cell protrusions composed of a highly branched actin filament meshwork assembled by the Arp2/3 complex. These structures not only hallmark the leading edge of cells adopting the adhesion-based mesenchymal mode of migration but are also thought to drive cell movement. Although regarded as textbook knowledge, the mechanism of formation of lamellipodia and ruffles has been revisited in the last years leveraging new technologies. Furthermore, recent observations have also challenged our current view of the function of lamellipodia and ruffles in mesenchymal cell migration. Here, I review this literature and compare it with older studies to highlight the controversies and the outstanding open issues in the field. Moreover, I outline simple and plausible explanations to reconcile conflicting results and conclusions. Finally, I integrate the mechanisms regulating actin-based protrusion in a unifying model that accounts for random and ballistic mesenchymal cell migration.
Keywords: Arp2/3 complex; actin; cancer; cell migration; formins; lamellipodia; ruffles.
Figures
Similar articles
-
CIP4 coordinates with phospholipids and actin-associated proteins to localize to the protruding edge and produce actin ribs and veils.J Cell Sci. 2013 Jun 1;126(Pt 11):2411-23. doi: 10.1242/jcs.117473. Epub 2013 Apr 9. J Cell Sci. 2013. PMID: 23572514 Free PMC article.
-
Efficiency of lamellipodia protrusion is determined by the extent of cytosolic actin assembly.Mol Biol Cell. 2017 May 15;28(10):1311-1325. doi: 10.1091/mbc.E16-05-0334. Epub 2017 Mar 22. Mol Biol Cell. 2017. PMID: 28331069 Free PMC article.
-
CRMP-1 enhances EVL-mediated actin elongation to build lamellipodia and the actin cortex.J Cell Biol. 2017 Aug 7;216(8):2463-2479. doi: 10.1083/jcb.201606084. Epub 2017 Jun 19. J Cell Biol. 2017. PMID: 28630144 Free PMC article.
-
Steering cell migration: lamellipodium dynamics and the regulation of directional persistence.Nat Rev Mol Cell Biol. 2014 Sep;15(9):577-90. doi: 10.1038/nrm3861. Nat Rev Mol Cell Biol. 2014. PMID: 25145849 Review.
-
Actin dynamics in cell migration.Essays Biochem. 2019 Oct 31;63(5):483-495. doi: 10.1042/EBC20190015. Essays Biochem. 2019. PMID: 31551324 Free PMC article. Review.
Cited by
-
ERK3/MAPK6 dictates CDC42/RAC1 activity and ARP2/3-dependent actin polymerization.Elife. 2023 Apr 14;12:e85167. doi: 10.7554/eLife.85167. Elife. 2023. PMID: 37057894 Free PMC article.
-
The Cycling of Intracellular Calcium Released in Response to Fluid Shear Stress Is Critical for Migration-Associated Actin Reorganization in Eosinophils.Cells. 2021 Jan 15;10(1):157. doi: 10.3390/cells10010157. Cells. 2021. PMID: 33467432 Free PMC article.
-
The dipeptide prolyl-hydroxyproline promotes cellular homeostasis and lamellipodia-driven motility via active β1-integrin in adult tendon cells.J Biol Chem. 2021 Jul;297(1):100819. doi: 10.1016/j.jbc.2021.100819. Epub 2021 May 23. J Biol Chem. 2021. PMID: 34029590 Free PMC article.
-
Reforming the Barrier: The Role of Formins in Wound Repair.Cells. 2022 Sep 6;11(18):2779. doi: 10.3390/cells11182779. Cells. 2022. PMID: 36139355 Free PMC article. Review.
-
Wound Healing from an Actin Cytoskeletal Perspective.Cold Spring Harb Perspect Biol. 2022 Aug 1;14(8):a041235. doi: 10.1101/cshperspect.a041235. Cold Spring Harb Perspect Biol. 2022. PMID: 35074864 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
