Lrp1 in osteoblasts controls osteoclast activity and protects against osteoporosis by limiting PDGF-RANKL signaling
- PMID: 29507818
- PMCID: PMC5826921
- DOI: 10.1038/s41413-017-0006-3
Lrp1 in osteoblasts controls osteoclast activity and protects against osteoporosis by limiting PDGF-RANKL signaling
Abstract
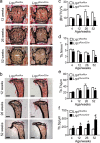
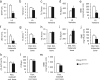
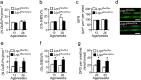
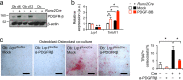
Skeletal health relies on architectural integrity and sufficient bone mass, which are maintained through a tightly regulated equilibrium of bone resorption by osteoclasts and bone formation by osteoblasts. Genetic studies have linked the gene coding for low-density lipoprotein receptor-related protein1 (Lrp1) to bone traits but whether these associations are based on a causal molecular relationship is unknown. Here, we show that Lrp1 in osteoblasts is a novel regulator of osteoclast activity and bone mass. Mice lacking Lrp1 specifically in the osteoblast lineage displayed normal osteoblast function but severe osteoporosis due to highly increased osteoclast numbers and bone resorption. Osteoblast Lrp1 limited receptor activator of NF-κB ligand (RANKL) expression in vivo and in vitro through attenuation of platelet-derived growth factor (PDGF-BB) signaling. In co-culture, Lrp1-deficient osteoblasts stimulated osteoclastogenesis in a PDGFRβ-dependent manner and in vivo treatment with the PDGFR tyrosine kinase inhibitor imatinib mesylate limited RANKL production and led to complete remission of the osteoporotic phenotype. These results identify osteoblast Lrp1 as a key regulator of osteoblast-to-osteoclast communication and bone mass through a PDGF-RANKL signaling axis in osteoblasts and open perspectives to further explore the potential of PDGF signaling inhibitors in counteracting bone loss as well as to evaluate the importance of functional LRP1 gene variants in the control of bone mass in humans.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
Imatinib promotes osteoblast differentiation by inhibiting PDGFR signaling and inhibits osteoclastogenesis by both direct and stromal cell-dependent mechanisms.J Bone Miner Res. 2007 Nov;22(11):1679-89. doi: 10.1359/jbmr.070719. J Bone Miner Res. 2007. PMID: 17663639
-
Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse.J Bone Miner Res. 2005 Sep;20(9):1659-68. doi: 10.1359/JBMR.050503. Epub 2005 May 2. J Bone Miner Res. 2005. PMID: 16059637
-
CTRP3 acts as a negative regulator of osteoclastogenesis through AMPK-c-Fos-NFATc1 signaling in vitro and RANKL-induced calvarial bone destruction in vivo.Bone. 2015 Oct;79:242-51. doi: 10.1016/j.bone.2015.06.011. Epub 2015 Jun 21. Bone. 2015. PMID: 26103094
-
Regulatory mechanisms of osteoblast and osteoclast differentiation.Oral Dis. 2002 May;8(3):147-59. doi: 10.1034/j.1601-0825.2002.01829.x. Oral Dis. 2002. PMID: 12108759 Review.
-
Osteoclast-Derived Extracellular Vesicles: Novel Regulators of Osteoclastogenesis and Osteoclast-Osteoblasts Communication in Bone Remodeling.Front Physiol. 2018 May 30;9:628. doi: 10.3389/fphys.2018.00628. eCollection 2018. Front Physiol. 2018. PMID: 29910740 Free PMC article. Review.
Cited by
-
Genome wide CRISPR screen for Pasteurella multocida toxin (PMT) binding proteins reveals LDL Receptor Related Protein 1 (LRP1) as crucial cellular receptor.PLoS Pathog. 2022 Dec 14;18(12):e1010781. doi: 10.1371/journal.ppat.1010781. eCollection 2022 Dec. PLoS Pathog. 2022. PMID: 36516199 Free PMC article.
-
Vindoline Inhibits RANKL-Induced Osteoclastogenesis and Prevents Ovariectomy-Induced Bone Loss in Mice.Front Pharmacol. 2020 Jan 22;10:1587. doi: 10.3389/fphar.2019.01587. eCollection 2019. Front Pharmacol. 2020. PMID: 32038256 Free PMC article.
-
Recombinant Human Peptide Growth Factors, Bone Morphogenetic Protein-7 (rhBMP7), and Platelet-Derived Growth Factor-BB (rhPDGF-BB) for Osteoporosis Treatment in an Oophorectomized Rat Model.Biomolecules. 2024 Mar 7;14(3):317. doi: 10.3390/biom14030317. Biomolecules. 2024. PMID: 38540737 Free PMC article.
-
The short-chain fatty acid receptors Gpr41/43 regulate bone mass by promoting adipogenic differentiation of mesenchymal stem cells.Front Endocrinol (Lausanne). 2024 Sep 19;15:1392418. doi: 10.3389/fendo.2024.1392418. eCollection 2024. Front Endocrinol (Lausanne). 2024. PMID: 39363899 Free PMC article.
-
Gut Microbiome Signature Are Correlated With Bone Mineral Density Alterations in the Chinese Elders.Front Cell Infect Microbiol. 2022 Mar 31;12:827575. doi: 10.3389/fcimb.2022.827575. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35433497 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

